Abstract
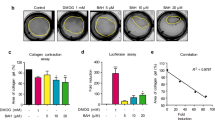
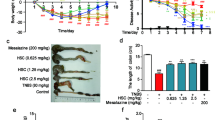
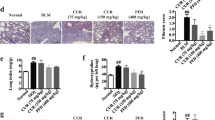
Intestinal fibrosis is a common complication of inflammatory bowel disease. There is still a lack of effective drugs for the prevention or treatment of intestinal fibrosis. Heat shock protein 47 (HSP47) plays a key role in the development of intestinal fibrosis. In this study we investigated the therapeutic potential and underlying mechanisms of fraxinellone, a degraded limonoid isolated from the root bark of Dictamnus dasycarpus, in the treatment of intestinal fibrosis. Intestinal fibrosis was induced in mice by dextran sodium sulfate (DSS) treatment. DDS-treated mice were administered fraxinellone (7.5, 15, 30 mg·kg−1·d−1, i.g.) for 45 days. We showed that fraxinellone administration dose-dependently alleviated DSS-induced intestinal impairments, and reduced the production of intestinal fibrosis biomarkers such as α-smooth muscle actin (SMA), collagen I, hydroxyproline, fibronectin and laminin, and cytokines such as TGF-β, TNF-α and IL-β. We then established in vitro intestinal fibrosis cell models in SW480 and HT-29 cells, and demonstrated that treatment with fraxinellone (3, 10, 30 μM) significantly relieved TGF-β-induced fibrosis responses by inhibiting the TGF-β/Smad2/3 signaling pathway. Molecular docking suggested that the fraxinellone might disrupt the interaction between HSP47 and collagen, which was confirmed by coimmunoprecipitation experiments. SPR analysis showed that fraxinellone had a high affinity for HSP47 with a Kd value of 3.542 × 10−5 M. This study provides a new example of HSP47-collagen intervention by a natural compound and has important implications for the clinical treatment of inflammation-induced issue fibrosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Bamias G, Pizarro TT, Cominelli F. Immunological regulation of intestinal fibrosis in inflammatory bowel disease. Inflamm Bowel Dis. 2022;28:337–49.
Silverstein MD, Loftus EV, Sandborn WJ, Tremaine WJ, Feagan BG, Nietert PJ, et al. Clinical course and costs of care for Crohn’s disease: Markov model analysis of a population-based cohort. Gastroenterology. 1999;117:49–57.
Gordon IO, Agrawal N, Willis E, Goldblum JR, Lopez R, Allende D, et al. Fibrosis in ulcerative colitis is directly linked to severity and chronicity of mucosal inflammation. Aliment Pharmacol Ther. 2018;47:922–39.
Henderson NC, Rieder F, Wynn TA. Fibrosis: from mechanisms to medicines. Nature. 2020;587:555–66.
Vallance BA, Gunawan MI, Hewlett B, Bercik P, Van Kampen C, Galeazzi F, et al. TGF-beta1 gene transfer to the mouse colon leads to intestinal fibrosis. Am J Physiol Gastrointest Liver Physiol. 2005;289:G116–28.
Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–61.
Amamou A, Yaker L, Leboutte M, Bole-Feysot C, Savoye G, Marion-Letellier R. Dietary AhR ligands have no anti-fibrotic properties in TGF-beta1-stimulated human colonic fibroblasts. Nutrients. 2022;14:3253.
Wang Y, Zhang Y, Lu B, Xi J, Ocansey DKW, Mao F, et al. hucMSC-Ex alleviates IBD-associated intestinal fibrosis by inhibiting ERK phosphorylation in intestinal fibroblasts. Stem Cells Int. 2023;2023:2828981.
Pallotta N, Barberani F, Hassan NA, Guagnozzi D, Vincoli G, Corazziari E. Effect of infliximab on small bowel stenoses in patients with Crohn’s disease. World J Gastroenterol. 2008;14:1885–90.
Calabrese E, Petruzziello C, Onali S, Condino G, Zorzi F, Pallone F, et al. Severity of postoperative recurrence in Crohn’s disease: correlation between endoscopic and sonographic findings. Inflamm Bowel Dis. 2009;15:1635–42.
Scheibe K, Kersten C, Schmied A, Vieth M, Primbs T, Carle B, et al. Inhibiting interleukin 36 receptor signaling reduces fibrosis in mice with chronic intestinal inflammation. Gastroenterology. 2019;156:1082–97.e11.
D’Haens G, Rieder F, Feagan BG, Higgins PDR, Panes J, Maaser C, et al. Challenges in the pathophysiology, diagnosis, and management of intestinal fibrosis in inflammatory bowel disease. Gastroenterology. 2022;162:26–31.
Ito S, Nagata K. Biology of Hsp47 (Serpin H1), a collagen-specific molecular chaperone. Semin Cell Dev Biol. 2017;62:142–51.
Niwa T, Kanamori T, Ueda T, Taguchi H. Global analysis of chaperone effects using a reconstituted cell-free translation system. Proc Natl Acad Sci USA. 2012;109:8937–42.
Zhu J, Xiong G, Fu H, Evers BM, Zhou BP, Xu R. Chaperone Hsp47 drives malignant growth and invasion by modulating an ECM gene network. Cancer Res. 2015;75:1580–91.
Widmer C, Gebauer JM, Brunstein E, Rosenbaum S, Zaucke F, Drogemuller C, et al. Molecular basis for the action of the collagen-specific chaperone Hsp47/SERPINH1 and its structure-specific client recognition. Proc Natl Acad Sci USA. 2012;109:13243–7.
Abd El-Fattah EE, Zakaria AY. Targeting HSP47 and HSP70: promising therapeutic approaches in liver fibrosis management. J Transl Med. 2022;20:544.
Bellaye PS, Burgy O, Bonniaud P, Kolb M. HSP47: a potential target for fibrotic diseases and implications for therapy. Expert Opin Ther Targets. 2021;25:49–62.
Bailly C, Vergoten G. Fraxinellone: From pesticidal control to cancer treatment. Pestic Biochem Physiol. 2020;168:104624.
Yoon JS, Yang H, Kim SH, Sung SH, Kim YC. Limonoids from Dictamnus dasycarpus protect against glutamate-induced toxicity in primary cultured rat cortical cells. J Mol Neurosci. 2010;42:9–16.
Wu XF, Ouyang ZJ, Feng LL, Chen G, Guo WJ, Shen Y, et al. Suppression of NF-kappaB signaling and NLRP3 inflammasome activation in macrophages is responsible for the amelioration of experimental murine colitis by the natural compound fraxinellone. Toxicol Appl Pharmacol. 2014;281:146–56.
Wu X, Wu X, Ma Y, Shao F, Tan Y, Tan T, et al. CUG-binding protein 1 regulates HSC activation and liver fibrogenesis. Nat Commun. 2016;7:13498.
Zheng B, Yuan M, Wang S, Tan Y, Xu Y, Ye J, et al. Fraxinellone alleviates kidney fibrosis by inhibiting CUG-binding protein 1-mediated fibroblast activation. Toxicol Appl Pharmacol. 2021;420:115530.
Wang J, Shi K, Li S, Chen L, Liu W, Wu X, et al. Meisoindigo attenuates dextran sulfate sodium-induced experimental colitis via its inhibition of TAK1 in macrophages. Int Immunopharmacol. 2021;101:108239.
Wang J, Shi K, An N, Li S, Bai M, Wu X, et al. Direct Inhibition of GSDMD by PEITC Reduces Hepatocyte Pyroptosis and Alleviates Acute Liver Injury in Mice. Front Immunol. 2022;13:825428.
Lu NH, Zhao HQ, Jing M, Liu X, Ren CZ, Liu XF, et al. The pharmacodynamic active components study of Tibetan medicine Gentianopsis paludosa on ulcerative colitis fibrosis. Int Immunopharmacol. 2017;46:163–9.
Daulagala AC, Kourtidis A. ECM Substrates Impact RNAi localization at adherens junctions of colon epithelial cells. Cells 2022;11:3740.
Vieujean S, Hu S, Bequet E, Salee C, Massot C, Bletard N, et al. Potential role of epithelial endoplasmic reticulum stress and anterior gradient protein 2 homologhomologue in Crohn’s disease fibrosis. J Crohns Colitis. 2021;15:1737–50.
Yu M, Wu H, Wang J, Chen X, Pan J, Liu P, et al. Vitamin D receptor inhibits EMT via regulation of the epithelial mitochondrial function in intestinal fibrosis. J Biol Chem. 2021;296:100531.
Wenxiu J, Mingyue Y, Fei H, Yuxin L, Mengyao W, Chenyang L, et al. Effect and mechanism of TL1A expression on epithelial-mesenchymal transition during chronic colitis-related intestinal fibrosis. Mediators Inflamm. 2021;2021:5927064.
Flier SN, Tanjore H, Kokkotou EG, Sugimoto H, Zeisberg M, Kalluri R. Identification of epithelial to mesenchymal transition as a novel source of fibroblasts in intestinal fibrosis. J Biol Chem. 2010;285:20202–12.
Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalingsignalling. Nature. 2003;425:577–84.
Cai H, Sasikumar P, Little G, Bihan D, Hamaia SW, Zhou A, et al. Identification of HSP47 binding site on native collagen and its implications for the development of HSP47 inhibitors. Biomolecules. 2021;11:983.
Rieder F, Fiocchi C. Intestinal fibrosis in IBD–a dynamic, multifactorial process. Nat Rev Gastroenterol Hepatol. 2009;6:228–35.
Wang J, Lin S, Brown JM, van Wagoner D, Fiocchi C, Rieder F. Novel mechanisms and clinical trial endpoints in intestinal fibrosis. Immunol Rev. 2021;302:211–27.
Shi J, Sun S, Xing S, Huang C, Huang Y, Wang Q, et al. Fraxinellone inhibits progression of glioblastoma by regulating via regulating the SIRT3 signaling pathway. Biomed Pharmacother. 2022;153:113416.
Kim MJ, Bae GS, Jo IJ, Choi SB, Kim DG, Jung HJ, et al. Fraxinellone inhibits inflammatory cell infiltration during acute pancreatitis by suppressing inflammasome activation. Int Immunopharmacol. 2019;69:169–77.
Wu F, Shao Q, Hu M, Zhao Y, Dong R, Fang K, et al. Wu-Mei-Wan ameliorates chronic colitis-associated intestinal fibrosis through inhibiting fibroblast activation. J Ethnopharmacol. 2020;252:112580.
Lovisa S, Genovese G, Danese S. Role of Epithelial-to-mesenchymal transition in inflammatory bowel disease. J Crohns Colitis. 2019;13:659–68.
Bataille F, Rohrmeier C, Bates R, Weber A, Rieder F, Brenmoehl J, et al. Evidence for a role of epithelial mesenchymal transition during pathogenesis of fistulae in Crohn’s disease. Inflamm Bowel Dis. 2008;14:1514–27.
Miyamura T, Sakamoto N, Kakugawa T, Taniguchi H, Akiyama Y, Okuno D, et al. Small molecule inhibitor of HSP47 prevents pro-fibrotic mechanisms of fibroblasts in vitro. Biochem Biophys Res Commun. 2020;530:561–5.
Abraham ET, Oecal S, Morgelin M, Schmid PWN, Buchner J, Baumann U, et al. Collagen’s primary structure determines collagen:HSP47 complex stoichiometry. J Biol Chem. 2021;297:101169.
Xie S, Xing Y, Shi W, Zhang M, Chen M, Fang W, et al. Cardiac fibroblast heat shock protein 47 aggravates cardiac fibrosis post myocardial ischemia-reperfusion injury by encouraging ubiquitin specific peptidase 10 dependent Smad4 deubiquitination. Acta Pharm Sin B. 2022;12:4138–53.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 82073910, 82173871, 21937005), the Open Fund of State Key Laboratory of Pharmaceutical Biotechnology, Nanjing University, China (Grant No. KF-GN-202101), and Fundamental Research Funds for the Central University (021414380503).
Author information
Authors and Affiliations
Contributions
JW, XFW, and QX designed research. JW, MB, CZ, NA, and LW performed research and analyzed data. XNW, RHD, YS, XDW, XFW, ZYY, and QX contributed new reagents. JW and XFW wrote the paper. All of the authors have approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, J., Bai, M., Zhang, C. et al. Natural compound fraxinellone ameliorates intestinal fibrosis in mice via direct intervention of HSP47-collagen interaction in the epithelium. Acta Pharmacol Sin 44, 2469–2478 (2023). https://doi.org/10.1038/s41401-023-01143-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-023-01143-1