Abstract
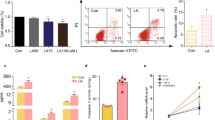
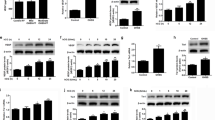
Polycystic ovary syndrome (PCOS) is a disorder with endocrinal and metabolic problems in reproductive aged women. Evidence shows that PCOS is in a high prone trend to develop kidney diseases. In this study, we investigated the mediators responsible for PCOS-related kidney injury. We found that tumor necrosis factor (TNF-α) levels were significantly increased in serum and primary cultured granulosa cells (GCs) from PCOS patients. Serum TNF-α levels were positively correlated with serum testosterone and luteinizing hormone (LH)/follicle-stimulating hormone (FSH) ratio, suggesting its positive role in the severity of PCOS. Serum TNF-α levels were also positively correlated with the levels of urinary KapU, LamU, α1‐MU and β2‐MU, the markers for renal tubular cell-derived proteinuria. We established a PCOS mouse model by resection of the right kidney, followed by daily administration of dihydrotestosterone (DHT, 27.5 μg, i.p.) from D7 for 90 days. We found that TNF-α levels were significantly increased in the ovary and serum of the mice, accompanied by increased renal tubular cell apoptosis, inflammation and fibrosis in kidneys. Furthermore, the receptor of TNF-α, tumor necrosis factor receptor 1 (TNFR1), was significantly upregulated in renal tubular cells. We treated human ovarian granulosa-like tumor cells (KGN) with DHT (1 μg/ml) in vitro, the conditioned medium derived from the granulosa cell culture greatly accelerated apoptotic injury in human proximal tubular epithelial cells (HKC-8), which was blocked after knockdown of TNF-α in KGN cells. Furthermore, knockdown of TNFR1 in renal tubular epithelial cells greatly ameliorated cell injury induced by granulosa cell-derived conditioned medium. These results suggest that serum TNF-α plays a key role in mediating inflammation and apoptosis in renal tubular cells associated with PCOS-related kidney injury.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Jones GL, Hall JM, Balen AH, Ledger WL. Health-related quality of life measurement in women with polycystic ovary syndrome: a systematic review. Hum Reprod Update. 2008;14:15–25.
Li R, Zhang Q, Yang D, Li S, Lu S, Wu X, et al. Prevalence of polycystic ovary syndrome in women in China: a large community-based study. Hum Reprod. 2013;28:2562–9.
Azziz R, Carmina E, Chen Z, Dunaif A, Laven JS, Legro RS, et al. Polycystic ovary syndrome. Nat Rev Dis Prim. 2016;2:16057.
Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-sponsored 3rd PCOS consensus workshop group. Fertil Steril. 2012;97:28–38.e25.
Stepto NK, Cassar S, Joham AE, Hutchison SK, Harrison CL, Goldstein RF, et al. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic-hyperinsulaemic clamp. Hum Reprod. 2013;28:777–84.
Gambineri A, Patton L, Altieri P, Pagotto U, Pizzi C, Manzoli L, et al. Polycystic ovary syndrome is a risk factor for type 2 diabetes: results from a long-term prospective study. Diabetes. 2012;61:2369–74.
Lim SS, Davies MJ, Norman RJ, Moran LJ. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2012;18:618–37.
Santoro N, Eisenberg E, Trussell JC, Craig LB, Gracia C, Huang H, et al. Fertility-related quality of life from two RCT cohorts with infertility: unexplained infertility and polycystic ovary syndrome. Hum Reprod. 2016;31:2268–79.
Moran LJ, Deeks AA, Gibson-Helm ME, Teede HJ. Psychological parameters in the reproductive phenotypes of polycystic ovary syndrome. Hum Reprod. 2012;27:2082–8.
Anderson SA, Barry JA, Hardiman PJ. Risk of coronary heart disease and risk of stroke in women with polycystic ovary syndrome: a systematic review and meta-analysis. Int J Cardiol. 2014;176:486–7.
Dumesic DA, Lobo RA. Cancer risk and PCOS. Steroids. 2013;78:782–5.
Ziaee A, Oveisi S, Ghorbani A, Hashemipour S, Mirenayat M. Association between metabolic syndrome and premicroalbuminuria among Iranian women with polycystic ovary syndrome: a case control study. Glob J Health Sci. 2012;5:187–92.
Gozukara IO, Gozukara KH, Kucur SK, Karakılıc EU, Keskin H, Akdeniz D, et al. Association of glomerular filtration rate with inflammation in polycystic ovary syndrome. Int J Fertil Steril. 2015;9:176–82.
Patil CN, Racusen LC, Reckelhoff JF. Consequences of advanced aging on renal function in chronic hyperandrogenemic female rat model: implications for aging women with polycystic ovary syndrome. Physiol Rep. 2017;5:e13461.
Song Y, Ye W, Ye H, Xie T, Shen W, Zhou L. Serum testosterone acts as a prognostic indicator in polycystic ovary syndrome-associated kidney injury. Physiol Rep. 2019;7:e14219.
Panidis D, Tziomalos K, Misichronis G, Papadakis E, Betsas G, Katsikis I, et al. Insulin resistance and endocrine characteristics of the different phenotypes of polycystic ovary syndrome: a prospective study. Hum Reprod. 2012;27:541–9.
Nautiyal H, Imam SS, Alshehri S, Ghoneim MM, Afzal M, Alzarea SI, et al. Polycystic ovarian syndrome: a complex disease with a genetics approach. Biomedicines. 2022;10:540.
Daan NM, Louwers YV, Koster MP, Eijkemans MJ, de Rijke YB, Lentjes EW, et al. Cardiovascular and metabolic profiles amongst different polycystic ovary syndrome phenotypes: who is really at risk? Fertil Steril. 2014;102:1444–51.e3.
Zhang HY, Guo CX, Zhu FF, Qu PP, Lin WJ, Xiong J. Clinical characteristics, metabolic features, and phenotype of Chinese women with polycystic ovary syndrome: a large-scale case-control study. Arch Gynecol Obstet. 2013;287:525–31.
Kauffman RP, Baker TE, Baker VM, DiMarino P, Castracane VD. Endocrine and metabolic differences among phenotypic expressions of polycystic ovary syndrome according to the 2003 Rotterdam consensus criteria. Am J Obstet Gynecol. 2008;198:670.e1–10.
Carmina E, Campagna AM, Lobo RA. A 20-year follow-up of young women with polycystic ovary syndrome. Obstet Gynecol. 2012;119:263–9.
Hayden MS, Ghosh S. Regulation of NF-kappaB by TNF family cytokines. Semin Immunol. 2014;26:253–66.
Horiuchi T, Mitoma H, Harashima S, Tsukamoto H, Shimoda T. Transmembrane TNF-alpha: structure, function and interaction with anti-TNF agents. Rheumatology. 2010;49:1215–28.
Yang S, Wang J, Brand DD, Zheng SG. Role of TNF-TNF receptor 2 signal in regulatory T Cells and its therapeutic implications. Front Immunol. 2018;9:784.
Borghi A, Verstrepen L, Beyaert R. TRAF2 multitasking in TNF receptor-induced signaling to NF-kappaB, MAP kinases and cell death. Biochem Pharmacol. 2016;116:1–10.
Barnabei L, Laplantine E, Mbongo W, Rieux-Laucat F, Weil R. NF-kappaB: at the borders of autoimmunity and inflammation. Front Immunol. 2021;12:716469.
Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1:a001651.
Gnanadass SA, Prabhu YD, Gopalakrishnan AV. Association of metabolic and inflammatory markers with polycystic ovarian syndrome (PCOS): an update. Arch Gynecol Obstet. 2021;303:631–43.
Dabravolski SA, Nikiforov NG, Eid AH, Nedosugova LV, Starodubova AV, Popkova TV, et al. Mitochondrial dysfunction and chronic inflammation in polycystic ovary syndrome. Int J Mol Sci. 2021;22:3923.
Zhai Y, Pang Y. Systemic and ovarian inflammation in women with polycystic ovary syndrome. J Reprod Immunol. 2022;151:103628.
Xiong YL, Liang XY, Yang X, Li Y, Wei LN. Low-grade chronic inflammation in the peripheral blood and ovaries of women with polycystic ovarian syndrome. Eur J Obstet Gynecol Reprod Biol. 2011;159:148–50.
Escobar-Morreale HF, Calvo RM, Sancho J, San Millán JL. TNF-alpha and hyperandrogenism: a clinical, biochemical, and molecular genetic study. J Clin Endocrinol Metab. 2001;86:3761–7.
Alissa EM, Algarni SA, Khaffji AJ, Al Mansouri NM. Role of inflammatory markers in polycystic ovaries syndrome: in relation to insulin resistance. J Obstet Gynaecol Res. 2021;47:1409–15.
Thathapudi S, Kodati V, Erukkambattu J, Katragadda A, Addepally U, Hasan Q. Tumor necrosis factor-alpha and polycystic ovarian syndrome: a clinical, biochemical, and molecular genetic study. Genet Test Mol Biomark. 2014;18:605–9.
Kordestani F, Mazloomi S, Mortazavi Y, Mazloomzadeh S, Fathi M, Rahmanpour H, et al. Preliminary study showing no association between G238A (rs361525) tumor necrosis factor-alpha (TNF-alpha) gene polymorphism and its serum level, hormonal and biochemical aspects of polycystic ovary syndrome. BMC Med Genet. 2018;19:149.
van Houten ELAF, Kramer P, McLuskey A, Karels B, Themmen APN, Visser JA. Reproductive and metabolic phenotype of a mouse model of PCOS. Endocrinology. 2012;153:2861–9.
Zhou L, Zhou S, Yang P, Tian Y, Feng Z, Xie XQ, et al. Targeted inhibition of the type 2 cannabinoid receptor is a novel approach to reduce renal fibrosis. Kidney Int. 2018;94:756–72.
Zhou L, Li Y, Hao S, Zhou D, Tan RJ, Nie J, et al. Multiple genes of the renin-angiotensin system are novel targets of Wnt/beta-catenin signaling. J Am Soc Nephrol. 2015;26:107–20.
Carmina E, Lobo RA. Comparing lean and obese PCOS in different PCOS phenotypes: evidence that the body weight is more important than the Rotterdam phenotype in influencing the metabolic status. Diagnostics. 2022;12:2313.
Johansson J, Stener-Victorin E. Polycystic ovary syndrome: effect and mechanisms of acupuncture for ovulation induction. Evid Based Complement Altern Med. 2013;2013:762615.
Balen AH, Laven JS, Tan SL, Dewailly D. Ultrasound assessment of the polycystic ovary: international consensus definitions. Hum Reprod Update. 2003;9:505–14.
Li Y, Chen C, Ma Y, Xiao J, Luo G, Li Y, et al. Multi-system reproductive metabolic disorder: significance for the pathogenesis and therapy of polycystic ovary syndrome (PCOS). Life Sci. 2019;228:167–75.
Pawelczak M, Rosenthal J, Milla S, Liu YH, Shah B. Evaluation of the pro-inflammatory cytokine tumor necrosis factor-alpha in adolescents with polycystic ovary syndrome. J Pediatr Adolesc Gynecol. 2014;27:356–9.
Goswami S, Choudhuri S, Bhattacharya B, Bhattacharjee R, Roy A, Mukhopadhyay S, et al. Chronic inflammation in polycystic ovary syndrome: a case-control study using multiple markers. Int J Reprod Biomed. 2021;19:313–20.
Lang Q, Yidong X, Xueguang Z, Sixian W, Wenming X, Tao Z. ETA-mediated anti-TNF-alpha therapy ameliorates the phenotype of PCOS model induced by letrozole. PLoS One. 2019;14:e0217495.
Stener-Victorin E, Padmanabhan V, Walters KA, Campbell RE, Benrick A, Giacobini P, et al. Animal models to understand the etiology and pathophysiology of polycystic ovary syndrome. Endocr Rev. 2020;41:bnaa010.
Mannerås L, Cajander S, Holmäng A, Seleskovic Z, Lystig T, Lönn M, et al. A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology. 2007;148:3781–91.
Caldwell ASL, Edwards MC, Desai R, Jimenez M, Gilchrist RB, Handelsman DJ, et al. Neuroendocrine androgen action is a key extraovarian mediator in the development of polycystic ovary syndrome. Proc Natl Acad Sci USA. 2017;114:E3334–43.
Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant 82225010, 82070707); Outstanding Youths Development Scheme of Nanfang Hospital, Southern Medical University (2019J013, 2021J001); the Presidential Foundation of Nanfang Hospital (Grant No. 2019Z006), and Guangdong Provincial Clinical Research Center for Kidney Disease (No. 2020B1111170013).
Author information
Authors and Affiliations
Contributions
LLZ conceived this study. LLZ and YLS participated in its design and coordination. HYY, WTY, CXX, JML, JHM, WWS and XLL conducted the experiments and contributed to data analysis and interpretation. HYY drafted the manuscript. LLZ helped to revise the manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ye, Hy., Song, Yl., Ye, Wt. et al. Serum granulosa cell-derived TNF-α promotes inflammation and apoptosis of renal tubular cells and PCOS-related kidney injury through NF-κB signaling. Acta Pharmacol Sin 44, 2432–2444 (2023). https://doi.org/10.1038/s41401-023-01128-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-023-01128-0
Keywords
This article is cited by
-
Butyrate alleviates renal inflammation and fibrosis in a rat model of polycystic ovarian syndrome by suppression of SDF-1
BMC Pharmacology and Toxicology (2023)