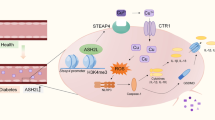
Abstract
Endothelial dysfunction, a central hallmark of cardiovascular pathogenesis in diabetes mellitus, is characterized by impaired endothelial nitric oxide synthase (eNOS) and NO bioavailability. However, the underlying mechanisms remain unclear. Here in this study, we aimed to identify the role of calmodulin (CaM) in diabetic eNOS dysfunction. Human umbilical vein endothelial cells and murine endothelial progenitor cells (EPCs) treated with high glucose (HG) exhibited downregulated CaM mRNA/protein and vascular endothelial growth factor (VEGF) expression with impeded eNOS phosphorylation and cell migration/tube formation. These perturbations were reduplicated in CALM1-knockdown cells but prevented in CALM1-overexpressing cells. EPCs from type 2 diabetes animals behaved similarly to HG-treated normal EPCs, which could be rescued by CALM1-gene transduction. Consistently, diabetic animals displayed impaired eNOS phosphorylation, endothelium-dependent dilation, and CaM expression in the aorta, as well as deficient physical interaction of CaM and eNOS in the gastrocnemius. Local CALM1 gene delivery into a diabetic mouse ischemic hindlimb improved the blunted limb blood perfusion and gastrocnemius angiogenesis, and foot injuries. Diabetic patients showed insufficient foot microvascular autoregulation, eNOS phosphorylation, and NO production with downregulated CaM expression in the arterial endothelium, and abnormal CALM1 transcription in genome-wide sequencing analysis. Therefore, our findings demonstrated that downregulated CaM expression is responsible for endothelium dysfunction and angiogenesis impairment in diabetes, and provided a novel mechanism and target to protect against diabetic endothelial injury.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan B, et al. IDF diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pr. 2021;183:109119.
Skyler J, Bakris G, Bonifacio E, Darsow T, Eckel R, Groop L, et al. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes. 2017;66:241–55.
Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–70.
Su J, Lucchesi PA, Gonzalez-Villalobos RA, Palen DI, Rezk BM, Suzuki Y, et al. Role of advanced glycation end products with oxidative stress in resistance artery dysfunction in type 2 diabetic mice. Arterioscler Thromb Vasc Biol. 2008;28:1432–8.
Förstermann U, Sessa W. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–37.
Abdo A, Rayner B, van Reyk D, Hawkins C. Low-density lipoprotein modified by myeloperoxidase oxidants induces endothelial dysfunction. Redox Biol. 2017;13:623–32.
Li F, Li Q, Shi X, Guo Y. Maslinic acid inhibits impairment of endothelial functions induced by high glucose in HAEC cells through improving insulin signaling and oxidative stress. Biomed Pharmacother. 2017;95:904–13.
Elrod J, Duranski M, Langston W, Greer J, Tao L, Dugas T, et al. eNOS gene therapy exacerbates hepatic ischemia-reperfusion injury in diabetes: a role for eNOS uncoupling. Circ Res. 2006;99:78–85.
Ying L, Li N, He Z, Zeng X, Nan Y, Chen J, et al. Fibroblast growth factor 21 ameliorates diabetes-induced endothelial dysfunction in mouse aorta via activation of the CaMKK2/AMPKα signaling pathway. Cell Death Dis. 2019;10:665.
Breton-Romero R, Feng B, Holbrook M, Farb MG, Fetterman JL, Linder EA, et al. Endothelial dysfunction in human diabetes is mediated by Wnt5a-JNK signaling. Arterioscler Thromb Vasc Biol. 2016;36:561–9.
Bharath L, Ruan T, Li Y, Ravindran A, Wan X, Nhan J, et al. Ceramide-initiated protein phosphatase 2A activation onctributes to arterial dysfunction in vivo. Diabetes. 2015;64:3914–26.
Kobayashi T, Nemoto S, Ishida K, Taguchi K, Matsumoto T, Kamata K. Involvement of CaM kinaseII in the impairment of endothelial function and eNOS activity in aortas of type 2 diabetic rats. Clin Sci (Lond). 2012;123:375–86.
Wong M, Samal A, Lee M, Vlach J, Novikov N, Niedziela-Majka A, et al. The KN-93 molecule inhibits calcium/calmodulin-dependent protein kinase II (CaMKII) activity by binding to Ca2+/CaM. J Mol Biol. 2019;426:1440–59.
Lu S, Liao Z, Lu X, Katschinski D, Mercola M, Chen J, et al. Hyperglycemia acutely increases cytosolic reactive oxygen species via -linked GlcNAcylation and CaMKII activation in mouse ventricular myocytes. Circ Res. 2020;126: e80–96.
Erickson J, Pereira L, Wang L, Han G, Ferguson A, Dao K, et al. Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation. Nature. 2013;502:372–6.
Zhang M, Song P, Xu J, Zou M. Activation of NAD(P)H oxidases by thromboxane A2 receptor uncouples endothelial nitric oxide synthase. Arterioscler Thromb Vasc Biol. 2011;31:125–32.
Piazza M, Guillemette J, Dieckmann T. Dynamics of nitric oxide synthase-calmodulin interactions at physiological calcium concentrations. Biochemistry. 2015;54:1989–2000.
Piazza M, Dieckmann T, Guillemette J. Structural studies of a complex between endothelial nitric oxide synthase and calmodulin at physiological calcium concentration. Biochemistry. 2016;55:5962–71.
Jung HJ, Kim JH, Shim JS, Kwon HJ. A novel Ca2+/calmodulin antagonist HBC inhibits angiogenesis and down-regulates hypoxia-inducible factor. J Biol Chem. 2010;285:25867–74.
Sarkar K, Fox-Talbot K, Steenbergen C, Bosch-Marcé M, Semenza GL. Adenoviral transfer of HIF-1 enhances vascular responses to critical limb ischemia in diabetic mice. Proc Natl Acad Sci USA. 2009;106:18769–74.
Shen W, Peng W, Shao Y, Xu J, Dai G, Zhang Y, et al. Localization and activity of calmodulin is involved in cell-cell adhesion of tumor cells and endothelial cells in response to hypoxic stress. Cell Biol Toxicol. 2007;23:323–35.
Berchtold MW, Villalobo A. The many faces of calmodulin in cell proliferation, programmed cell death, autophagy, and cancer. Biochim Biophys Acta. 2014;1843:398–435.
Tidow H, Nissen P. Structural diversity of calmodulin binding to its target sites. FEBS J. 2013;280:5551–65.
Chen Z, Ding L, Yang W, Wang J, Chen L, Chang Y, et al. Hepatic activation of the FAM3C-HSF1-CaM pathway attenuates hyperglycemia of obese diabetic mice. Diabetes. 2017;66:1185–97.
Pan R, Lou J, Wei L. Significant effects of Ganoderma lucidum polysaccharide on lipid metabolism in diabetes may be associated with the activation of the FAM3C‑HSF1‑CAM signaling pathway. Exp Ther Med. 2021;22:820.
Cui W, Zheng Y, Zhang Q, Wang J, Wang L, Yang W, et al. Low-molecular-weight fucoidan protects endothelial function and ameliorates basal hypertension in diabetic Goto-Kakizaki rats. Lab Invest. 2014;94:382–93.
Wang K, Dai X, He J, Yan X, Yang C, Fan X, et al. Endothelial overexpression of metallothionein prevents diabetes-induced impairment in ischemia angiogenesis through preservation of HIF-1α/SDF-1/VEGF signaling in endothelial progenitor cells. Diabetes. 2020;69:1779–92.
Wils J, Favre J, Bellien J. Modulating putative endothelial progenitor cells for the treatment of endothelial dysfunction and cardiovascular complications in diabetes. Pharmacol Ther. 2017;170:98–115.
Dai X, Yan X, Zeng J, Chen J, Wang Y, Chen J, et al. Elevating CXCR7 improves angiogenic function of EPCs via Akt/GSK-3beta/Fyn-mediated Nrf2 activation in diabetic limb ischemia. Circ Res. 2017;120:e7–e23.
Meng S, Lv J, Chanda PK, Owusu I, Chen K, Cooke JP. Reservoir of fibroblasts promotes recovery from limb ischemia. Circulation. 2020;142:1647–62.
Bir S, Pattillo C, Pardue S, Kolluru G, Shen X, Giordano T, et al. Nitrite anion therapy protects against chronic ischemic tissue injury in db/db diabetic mice in a NO/VEGF-dependent manner. Diabetes. 2014;63:270–81.
Shah M, Brownlee M. Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ Res. 2016;118:1808–29.
Wang W, Ha C, Jhun B, Wong C, Jain M, Jin Z. Fluid shear stress stimulates phosphorylation-dependent nuclear export of HDAC5 and mediates expression of KLF2 and eNOS. Blood. 2010;115:2971–9.
Sriram K, Laughlin J, Rangamani P, Tartakovsky D. Shear-induced nitric oxide production by endothelial cells. Biophys J. 2016;111:208–21.
Crotti L, Johnson C, Graf E, De Ferrari G, Cuneo B, Ovadia M, et al. Calmodulin mutations associated with recurrent cardiac arrest in infants. Circulation. 2013;127:1009–17.
Jensen H, Brohus M, Nyegaard M, Overgaard M. Human calmodulin mutations. Front Mol Neurosci. 2018;11:396.
Hegyi B, Bers D, Bossuyt J. CaMKII signaling in heart diseases: emerging role in diabetic cardiomyopathy. J Mol Cell Cardiol. 2019;127:246–59.
Kolluru G, Bir S, Kevil C. Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing. Int J Vasc Med. 2012;2012:918267.
Persechini A, Stemmer P. Calmodulin is a limiting factor in the cell. Trends Cardiovasc Med. 2002;12:32–7.
Tran Q, Black D, Persechini A. Intracellular coupling via limiting calmodulin. J Biol Chem. 2003;278:24247–50.
Oztürk Y, Aydin S, Altan VM, Yildizoğlu-ari N, Ozçelikay AT. Effect of short and long term streptozotocin diabetes on smooth muscle calmodulin levels in the rat. Cell Calcium. 1994;16:81–6.
Paul S, Ali A, Katare R. Molecular complexities underlying the vascular complications of diabetes mellitus-A comprehensive review. J Diabetes Complicat. 2020;34:107613.
Fadini G. A reappraisal of the role of circulating (progenitor) cells in the pathobiology of diabetic complications. Diabetologia. 2014;57:4–15.
Gambardella J, Khondkar W, Morelli M, Wang X, Santulli G, Trimarco V. Arginine and endothelial function. Biomedicines. 2020;8:277.
Cai H, Liu D, Garcia JG. CaM Kinase II-dependent pathophysiological signalling in endothelial cells. Cardiovasc Res. 2008;77:30–4.
Yasuda R, Hayashi Y, Hell JW. CaMKII: a central molecular organizer of synaptic plasticity, learning and memory. Nat Rev Neurosci. 2022;23:666–82.
Bhattacharyya M, Stratton MM, Going CC, McSpadden ED, Huang Y, Susa AC, et al. Molecular mechanism of activation-triggered subunit exchange in Ca2+/calmodulin-dependent protein kinase II. Elife. 2016;5:e13405.
Schalkwijk CG, Stehouwer CDA. Methylglyoxal, a highly reactive dicarbonyl compound, in diabetes, its vascular complications, and other age-related diseases. Physiol Rev. 2020;100:407–61.
Botusan I, Sunkari V, Savu O, Catrina A, Grünler J, Lindberg S, et al. Stabilization of HIF-1alpha is critical to improve wound healing in diabetic mice. Proc Natl Acad Sci USA. 2008;105:19426–31.
Ceradini D, Yao D, Grogan R, Callaghan M, Edelstein D, Brownlee M, et al. Decreasing intracellular superoxide corrects defective ischemia-induced new vessel formation in diabetic mice. J Biol Chem. 2008;283:10930–8.
Gunton JE. Hypoxia-inducible factors and diabetes. J Clin Invest. 2020;130:5063–73.
Acknowledgements
We thank Dr. Jin-huan Gao from Xuanwu Hospital, Capital Medical University for technical support in the establishment of hind limb ischemia model. This study was supported by the National Natural Science Foundation of China (81570206 and 81970197), and the Scientific Research Key Program of Beijing Municipal Commission of Education (KZ201710025023).
Author information
Authors and Affiliations
Contributions
TTL, HHX, and ZJL performed the research and wrote the manuscript. HPZ, ZXZ and HTZ prepared patient samples and collected information. ZQW, JYX, QL, YM and HJY provided research material and techniques and contributed to data interpretation. DLL directed the project, wrote, reviewed, and edited the manuscript. DLL is the guarantor of this project and, as such, has full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Tt., Xu, Hh., Liu, Zj. et al. Downregulated calmodulin expression contributes to endothelial cell impairment in diabetes. Acta Pharmacol Sin 44, 2492–2503 (2023). https://doi.org/10.1038/s41401-023-01127-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-023-01127-1
Keywords
This article is cited by
-
Deficiency of neutral cholesterol ester hydrolase 1 (NCEH1) impairs endothelial function in diet-induced diabetic mice
Cardiovascular Diabetology (2024)