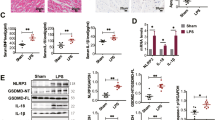
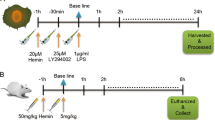
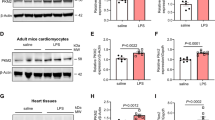
Abstract
Clinically, cardiac dysfunction is a key component of sepsis-induced multi-organ failure. Mitochondria are essential for cardiomyocyte homeostasis, as disruption of mitochondrial dynamics enhances mitophagy and apoptosis. However, therapies targeted to improve mitochondrial function in septic patients have not been explored. Transcriptomic data analysis revealed that the peroxisome proliferator-activated receptor (PPAR) signaling pathway in the heart was the most significantly decreased in the cecal ligation puncture-treated mouse heart model, and PPARα was the most notably decreased among the three PPAR family members. Male Pparafl/fl (wild-type), cardiomyocyte-specific Ppara-deficient (PparaΔCM), and myeloid-specific Ppara-deficient (PparaΔMac) mice were injected intraperitoneally with lipopolysaccharide (LPS) to induce endotoxic cardiac dysfunction. PPARα signaling was decreased in LPS-treated wild-type mouse hearts. To determine the cell type in which PPARα signaling was suppressed, the cell type-specific Ppara-null mice were examined. Cardiomyocyte- but not myeloid-specific Ppara deficiency resulted in exacerbated LPS-induced cardiac dysfunction. Ppara disruption in cardiomyocytes augmented mitochondrial dysfunction, as revealed by damaged mitochondria, lowered ATP contents, decreased mitochondrial complex activities, and increased DRP1/MFN1 protein levels. RNA sequencing results further showed that cardiomyocyte Ppara deficiency potentiated the impairment of fatty acid metabolism in LPS-treated heart tissue. Disruption of mitochondrial dynamics resulted in increased mitophagy and mitochondrial-dependent apoptosis in Ppara△CM mice. Moreover, mitochondrial dysfunction caused an increase of reactive oxygen species, leading to increased IL-6/STAT3/NF-κB signaling. 3-Methyladenine (3-MA, an autophagosome formation inhibitor) alleviated cardiomyocyte Ppara disruption-induced mitochondrial dysfunction and cardiomyopathy. Finally, pre-treatment with the PPARα agonist WY14643 lowered mitochondrial dysfunction-induced cardiomyopathy in hearts from LPS-treated mice. Thus, cardiomyocyte but not myeloid PPARα protects against septic cardiomyopathy by improving fatty acid metabolism and mitochondrial dysfunction, thus highlighting that cardiomyocyte PPARα may be a therapeutic target for the treatment of cardiac disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
References
Cecconi M, Evans L, Levy M, Rhodes A. Sepsis and septic shock. Lancet 2018;392:75–87.
Lelubre C, Vincent JL. Mechanisms and treatment of organ failure in sepsis. Nat Rev Nephrol. 2018;14:417–27.
Peoples JN, Saraf A, Ghazal N, Pham TT, Kwong JQ. Mitochondrial dysfunction and oxidative stress in heart disease. Exp Mol Med. 2019;51:162.
Picca A, Mankowski RT, Burman JL, Donisi L, Kim JS, Marzetti E, et al. Mitochondrial quality control mechanisms as molecular targets in cardiac ageing. Nat Rev Cardiol. 2018;15:543–54.
Onishi M, Yamano K, Sato M, Matsuda N, Okamoto K. Molecular mechanisms and physiological functions of mitophagy. EMBO J. 2021;40:e104705.
Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–52.
Bock FJ, Tait SWG. Mitochondria as multifaceted regulators of cell death. Nat Rev Mol Cell Biol. 2020;21:85–100.
Patoli D, Mignotte F, Deckert V, Dusuel A, Dumont A, Rieu A, et al. Inhibition of mitophagy drives macrophage activation and antibacterial defense during sepsis. J Clin Invest. 2020;130:5858–74.
Hussain T, Tan B, Yin Y, Blachier F, Tossou MC, Rahu N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid Med Cell Longev. 2016;2016:7432797.
Bougarne N, Weyers B, Desmet SJ, Deckers J, Ray DW, Staels B, et al. Molecular Actions of PPARalpha in lipid metabolism and inflammation. Endocr Rev. 2018;39:760–802.
Barlaka E, Galatou E, Mellidis K, Ravingerova T, Lazou A. Role of pleiotropic properties of peroxisome proliferator-activated receptors in the heart: focus on the nonmetabolic effects in cardiac protection. Cardiovasc Ther. 2016;34:37–48.
Young ME, Laws FA, Goodwin GW, Taegtmeyer H. Reactivation of peroxisome proliferator-activated receptor alpha is associated with contractile dysfunction in hypertrophied rat heart. J Biol Chem. 2001;276:44390–5.
Kaimoto S, Hoshino A, Ariyoshi M, Okawa Y, Tateishi S, Ono K, et al. Activation of PPAR-alpha in the early stage of heart failure maintained myocardial function and energetics in pressure-overload heart failure. Am J Physiol Heart Circ Physiol. 2017;312:H305–H313.
Standage SW, Bennion BG, Knowles TO, Ledee DR, Portman MA, McGuire JK, et al. PPARalpha augments heart function and cardiac fatty acid oxidation in early experimental polymicrobial sepsis. Am J Physiol Heart Circ Physiol. 2017;312:H239–H249.
Wang X, Zhu XX, Jiao SY, Qi D, Yu BQ, Xie GM, et al. Cardiomyocyte peroxisome proliferator-activated receptor alpha is essential for energy metabolism and extracellular matrix homeostasis during pressure overload-induced cardiac remodeling. Acta Pharmacol Sin. 2022;43:1231–42.
Brocker CN, Yue J, Kim D, Qu A, Bonzo JA, Gonzalez FJ. Hepatocyte-specific PPARA expression exclusively promotes agonist-induced cell proliferation without influence from nonparenchymal cells. Am J Physiol Gastrointest Liver Physiol. 2017;312:G283–G299.
Zhao H, Zhang M, Zhou F, Cao W, Bi L, Xie Y, et al. Cinnamaldehyde ameliorates LPS-induced cardiac dysfunction via TLR4-NOX4 pathway: The regulation of autophagy and ROS production. J Mol Cell Cardiol. 2016;101:11–24.
Qi D, Wei M, Jiao S, Song Y, Wang X, Xie G, et al. Hypoxia inducible factor 1alpha in vascular smooth muscle cells promotes angiotensin II-induced vascular remodeling via activation of CCL7-mediated macrophage recruitment. Cell Death Dis. 2019;10:544.
Busch K, Kny M, Huang N, Klassert TE, Stock M, Hahn A, et al. Inhibition of the NLRP3/IL-1beta axis protects against sepsis-induced cardiomyopathy. J Cachexia Sarcopenia Muscle. 2021;12:1653–68.
Li N, Zhou H, Wu H, Wu Q, Duan M, Deng W, et al. STING-IRF3 contributes to lipopolysaccharide-induced cardiac dysfunction, inflammation, apoptosis and pyroptosis by activating NLRP3. Redox Biol. 2019;24:101215.
Bhagat A, Shrestha P, Kleinerman ES. The innate immune system in cardiovascular diseases and its role in doxorubicin-induced cardiotoxicity. Int J Mol Sci. 2022;23:14649.
Ouyang H, Li Q, Zhong J, Xia F, Zheng S, Lu J, et al. Combination of melatonin and irisin ameliorates lipopolysaccharide-induced cardiac dysfunction through suppressing the Mst1-JNK pathways. J Cell Physiol. 2020;235:6647–59.
Abate M, Festa A, Falco M, Lombardi A, Luce A, Grimaldi A, et al. Mitochondria as playmakers of apoptosis, autophagy and senescence. Semin Cell Dev Biol. 2020;98:139–53.
Boengler K, Kosiol M, Mayr M, Schulz R, Rohrbach S. Mitochondria and ageing: role in heart, skeletal muscle and adipose tissue. J Cachexia Sarcopenia Muscle. 2017;8:349–69.
Wajner M, Amaral AU. Mitochondrial dysfunction in fatty acid oxidation disorders: insights from human and animal studies. Biosci Rep. 2015;36:e00281.
Shirihai OS, Song M, Dorn GW 2nd. How mitochondrial dynamism orchestrates mitophagy. Circ Res. 2015;116:1835–49.
Sun K, Jing X, Guo J, Yao X, Guo F. Mitophagy in degenerative joint diseases. Autophagy. 2021;17:2082–92.
Hu L, Huang B, Bai S, Tan J, Liu Y, Chen H, et al. SO2 derivatives induce dysfunction in human trophoblasts via inhibiting ROS/IL-6/STAT3 pathway. Ecotoxicol Environ Saf. 2021;210:111872.
Zanders L, Kny M, Hahn A, Schmidt S, Wundersitz S, Todiras M, et al. Sepsis induces interleukin 6, gp130/JAK2/STAT3, and muscle wasting. J Cachexia Sarcopenia Muscle. 2022;13:713–27.
Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell. 2011;21:92–101.
Montaigne D, Butruille L, Staels B. PPAR control of metabolism and cardiovascular functions. Nat Rev Cardiol. 2021;18:809–23.
Standage SW, Waworuntu RL, Delaney MA, Maskal SM, Bennion BG, Duffield JS, et al. Nonhematopoietic peroxisome proliferator-activated receptor-alpha protects against cardiac injury and enhances survival in experimental polymicrobial sepsis. Crit Care Med. 2016;44:e594–603.
Makrecka-Kuka M, Korzh S, Vilks K, Vilskersts R, Cirule H, Dambrova M, et al. Mitochondrial function in the kidney and heart, but not the brain, is mainly altered in an experimental model of endotoxaemia. Shock. 2019;52:e153–e162.
Chan DC. Mitochondrial dynamics and its involvement in disease. Annu Rev Pathol. 2020;15:235–59.
Giacomello M, Pyakurel A, Glytsou C, Scorrano L. The cell biology of mitochondrial membrane dynamics. Nat Rev Mol Cell Biol. 2020;21:204–24.
Lopaschuk GD, Karwi QG, Tian R, Wende AR, Abel ED. Cardiac energy metabolism in heart failure. Circ Res. 2021;128:1487–513.
Scarpulla RC. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev. 2008;88:611–38.
Chen M, Chen Z, Wang Y, Tan Z, Zhu C, Li Y, et al. Mitophagy receptor FUNDC1 regulates mitochondrial dynamics and mitophagy. Autophagy. 2016;12:689–702.
Doblado L, Lueck C, Rey C, Samhan-Arias AK, Prieto I, Stacchiotti A, et al. Mitophagy in human diseases. Int J Mol Sci. 2021;22:3903.
Feng Y, Madungwe NB, da Cruz Junho CV, Bopassa JC. Activation of G protein-coupled oestrogen receptor 1 at the onset of reperfusion protects the myocardium against ischemia/reperfusion injury by reducing mitochondrial dysfunction and mitophagy. Br J Pharmacol. 2017;174:4329–44.
Nguyen TN, Padman BS, Lazarou M. Deciphering the molecular signals of PINK1/Parkin mitophagy. Trends Cell Biol. 2016;26:733–44.
Miller S, Oleksy A, Perisic O, Williams RL. Finding a fitting shoe for Cinderella: Searching for an autophagy inhibitor. Autophagy. 2010;6:805–7.
Drosatos K, Khan RS, Trent CM, Jiang H, Son NH, Blaner WS, et al. Peroxisome proliferator-activated receptor-gamma activation prevents sepsis-related cardiac dysfunction and mortality in mice. Circ Heart Fail. 2013;6:550–62.
Acknowledgements
This study was supported by National Key R&D Program of China (2021YFA0805100), National Natural Science Foundation of China (82070474), Support Project of High-level Teachers in Beijing Municipal Universities in the Period of 13th Five-year Plan (CIT&TCD20190332), The Key Science and Technology Project of Beijing Municipal Institutions (KZ202010025032) to AJQ, National Natural Science Foundation of China (81800233) to XW. and the National Cancer Institute Intramural Research Program to FJG.
Author information
Authors and Affiliations
Contributions
XXZ designed the study, performed the experiments, and wrote the manuscript. XW, SYJ, YL, LS, YEC, QZ, YTS, and MW performed the experiments and analyzed the data. QX participated in the echocardiography. JJW was in charge of mice. JD and BQY participated in the initial elaboration of the project. JF provided transcription data and involved in writing the manuscript. FJG designed the experimental plan, supervised the study and involved in writing the manuscript. AJQ conceived, supervised the study, and wrote the manuscript. All authors read and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, Xx., Wang, X., Jiao, Sy. et al. Cardiomyocyte peroxisome proliferator-activated receptor α prevents septic cardiomyopathy via improving mitochondrial function. Acta Pharmacol Sin 44, 2184–2200 (2023). https://doi.org/10.1038/s41401-023-01107-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-023-01107-5