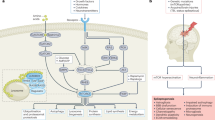
Abstract
Epilepsy is not well controlled by current anti-seizure drugs (ASDs). High mobility group box 1 (HMGB1) is a DNA-binding protein in the nucleus regulating transcriptional activity and maintaining chromatin structure and DNA repair. In epileptic brains, HMGB1 is released by activated glia and neurons, interacting with various receptors like Toll-like receptor 4 (TLR4) and downstream glutamatergic NMDA receptor, thus enhancing neural excitability. But there is a lack of small-molecule drugs targeting the HMGB1-related pathways. In this study we evaluated the therapeutic potential of inflachromene (ICM), an HMGB-targeting small-molecule inhibitor, in mouse epilepsy models. Pentylenetetrazol-, kainic acid- and kindling-induced epilepsy models were established in mice. The mice were pre-treated with ICM (3, 10 mg/kg, i.p.). We showed that ICM pretreatment significantly reduced the severity of epileptic seizures in all the three epilepsy models. ICM (10 mg/kg) exerted the most apparent anti-seizure effect in kainic acid-induced epileptic status (SE) model. By immunohistochemical analysis of brain sections from kainic acid-induced SE mice, we found that kainic acid greatly enhanced HMGB1 translocation in the hippocampus, which was attenuated by ICM pretreatment in subregion- and cell type-dependent manners. Notably, in CA1 region, the seizure focus, ICM pretreatment mainly inhibited HMGB1 translocation in microglia. Furthermore, the anti-seizure effect of ICM was related to HMGB1 targeting, as pre-injection of anti-HMGB1 monoclonal antibody (5 mg/kg, i.p.) blocked the seizure-suppressing effect of ICM in kainic acid-induced SE model. In addition, ICM pretreatment significantly alleviated pyramidal neuronal loss and granule cell dispersion in kainic acid-induced SE model. These results demonstrate that ICM is an HMGB-targeting small molecule with anti-seizure potential, which may help develop a potential drug for treating epilepsy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Devinsky O, Vezzani A, O’Brien TJ, Jette N, Scheffer IE, de Curtis M, et al. Epilepsy. Nat Rev Dis Prim. 2018;4:18024.
Wang Y, Chen Z. An update for epilepsy research and antiepileptic drug development: Toward precise circuit therapy. Pharmacol Ther. 2019;201:77–93.
Zienowicz M, Wisłowska A, Lehner M, Taracha E, Skórzewska A, Maciejak P, et al. The effect of fluoxetine in a model of chemically induced seizures-behavioral and immunocytochemical study. Neurosci Lett. 2005;373:226–31.
Jobst BC, Cascino GD. Resective epilepsy surgery for drug-resistant focal epilepsy: A review. JAMA 2015;313:285–93.
Mandke P, Vasquez KM. Interactions of high mobility group box protein 1 (HMGB1) with nucleic acids: Implications in DNA repair and immune responses. DNA Repair (Amst). 2019;83:102701.
Seong S-Y, Matzinger P. Hydrophobicity: An ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol. 2004;4:469–78.
Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139–62.
Paudel YN, Semple BD, Jones NC, Othman I, Shaikh MF. High mobility group box 1 (HMGB1) as a novel frontier in epileptogenesis: from pathogenesis to therapeutic approaches. J Neurochem. 2019;151:542–57.
Li YJ, Wang L, Zhang B, Gao F, Yang CM. Glycyrrhizin, an HMGB1 inhibitor, exhibits neuroprotective effects in rats after lithium-pilocarpine-induced status epilepticus. J Pharm Pharmacol. 2019;71:390–9.
Li C, Peng S, Liu X, Han C, Wang X, Jin T, et al. Glycyrrhizin, a direct HMGB1 antagonist, ameliorates inflammatory infiltration in a model of autoimmune thyroiditis via inhibition of TLR2-HMGB1 signaling. Thyroid 2017;27:722–31.
Zhao J, Wang Y, Xu C, Liu K, Wang Y, Chen L, et al. Therapeutic potential of an anti-high mobility group box-1 monoclonal antibody in epilepsy. Brain Behav Immun. 2017;64:308–19.
Lee S, Nam Y, Koo JY, Lim D, Park J, Ock J, et al. A small molecule binding HMGB1 and HMGB2 inhibits microglia-mediated neuroinflammation. Nat Chem Biol. 2014;10:1055–60.
Cho W, Koo JY, Park Y, Oh K, Lee S, Song JS, et al. Treatment of sepsis pathogenesis with High Mobility Group Box Protein 1-regulating anti-inflammatory agents. J Med Chem. 2017;60:170–9.
Lüttjohann A, Fabene PF, van Luijtelaar G. A revised Racine’s scale for PTZ-induced seizures in rats. Physiol Behav. 2009;98:579–86.
Wang Y, Xu C, Xu Z, Ji C, Liang J, Wang Y, et al. Depolarized GABAergic signaling in subicular microcircuits mediates generalized seizure in temporal lobe epilepsy. Neuron. 2017;95:92–105.e5.
Wang Y, Wang Y, Xu C, Wang S, Tan N, Chen C, et al. Direct septum-hippocampus cholinergic circuit attenuates seizure through driving somatostatin inhibition. Biol Psychiatry. 2020;87:843–56.
Xu Z, Wang Y, Chen B, Xu C, Wu X, Wang Y, et al. Entorhinal principal neurons mediate brain-stimulation treatments for epilepsy. EBioMedicine. 2016;14:148–60.
Paxinos G, Franklin KB. Paxinos and Franklin’s the mouse brain in stereotaxic coordinates. (Academic press, 2019).
Lévesque M, Avoli M. The kainic acid model of temporal lobe epilepsy. Neurosci Biobehav Rev. 2013;37:2887–99.
Xu C, Zhang S, Gong Y, Nao J, Shen Y, Tan B, et al. Subicular caspase-1 contributes to pharmacoresistance in temporal lobe epilepsy. Ann Neurol. 2021;90:377–90.
Wang Y, Shen Y, Cai X, Yu J, Chen C, Tan B, et al. Deep brain stimulation in the medial septum attenuates temporal lobe epilepsy via entrainment of hippocampal theta rhythm. CNS Neurosci Ther. 2021;27:577–86.
Hu TT, Yu J, Liu K, Du Y, Qu FH, Guo F, et al. A crucial role of HMGB1 in orofacial and widespread pain sensitization following partial infraorbital nerve transection. Brain Behav Immun. 2020;88:114–24.
Du Y, Xu CL, Yu J, Liu K, Lin SD, Hu TT, et al. HMGB1 in the mPFC governs comorbid anxiety in neuropathic pain. J Headache Pain. 2022;23:102.
Raol YH, Brooks-Kayal AR. Experimental models of seizures and epilepsies. Prog Mol Biol Transl Sci. 2012;105:57–82.
Zhao J, Zheng Y, Liu K, Chen J, Lai N, Fei F, et al. HMGB1 Is a therapeutic target and biomarker in diazepam-refractory status epilepticus with wide time window. Neurotherapeutics. 2020;17:710–21.
Morin F, Beaulieu C, Lacaille JC. Selective loss of GABA neurons in area CA1 of the rat hippocampus after intraventricular kainate. Epilepsy Res. 1998;32:363–9.
Devi PU, Manocha A, Vohora D. Seizures, antiepileptics, antioxidants and oxidative stress: an insight for researchers. Expert Opin Pharmacother. 2008;9:3169–77.
Brines ML, Sundaresan S, Spencer DD, de Lanerolle NC. Quantitative autoradiographic analysis of ionotropic glutamate receptor subtypes in human temporal lobe epilepsy: up-regulation in reorganized epileptogenic hippocampus. Eur J Neurosci. 1997;9:2035–44.
Samokhina E, Samokhin A. Neuropathological profile of the pentylenetetrazol (PTZ) kindling model. Int J Neurosci. 2018;128:1086–96.
Sharma AK, Reams RY, Jordan WH, Miller MA, Thacker HL, Snyder PW. Mesial temporal lobe epilepsy: Pathogenesis, induced rodent models and lesions. Toxicol Pathol. 2007;35:984–99.
Ramanjaneyulu R, Ticku MK. Interactions of pentamethylenetetrazole and tetrazole analogues with the picrotoxinin site of the benzodiazepine-GABA receptor-ionophore complex. Eur J Pharmacol. 1984;98:337–45.
Martin D, McNamara JO, Nadler JV. Kindling enhances sensitivity of CA3 hippocampal pyramidal cells to NMDA. J Neurosci. 1992;12:1928–35.
Luo L, Jin Y, Kim ID, Lee JK. Glycyrrhizin suppresses HMGB1 inductions in the hippocampus and subsequent accumulation in serum of a kainic acid-induced seizure mouse model. Cell Mol Neurobiol. 2014;34:987–97.
Li Z, Li B, Zhu X, Yin P, Liu J, Huang S, et al. Neuroprotective effects of anti-high-mobility group box 1 antibody in juvenile rat hippocampus after kainic acid-induced status epilepticus. Neuroreport. 2013;24:785–90.
Maroso M, Balosso S, Ravizza T, Liu J, Aronica E, Iyer AM, et al. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med. 2010;16:413–9.
Fu L, Liu K, Wake H, Teshigawara K, Yoshino T, Takahashi H, et al. Therapeutic effects of anti-HMGB1 monoclonal antibody on pilocarpine-induced status epilepticus in mice. Sci Rep. 2017;7:1179.
Maroso M, Balosso S, Ravizza T, Liu J, Bianchi ME, Vezzani A. Interleukin-1 type 1 receptor/Toll-like receptor signalling in epilepsy: the importance of IL-1beta and high-mobility group box 1. J Intern Med. 2011;270:319–26.
Nass RD, Wagner M, Surges R, Holdenrieder S. Time courses of HMGB1 and other inflammatory markers after generalized convulsive seizures. Epilepsy Res. 2020;162:106301.
Ruhnau J, Tennigkeit J, Ceesay S, Koppe C, Muszelewski M, Grothe S, et al. Immune alterations following neurological disorders: a comparison of stroke and seizures. Front Neurol. 2020;11:425.
Akahoshi N, Murashima YL, Himi T, Ishizaki Y, Ishii I. Increased expression of the lysosomal protease cathepsin S in hippocampal microglia following kainate-induced seizures. Neurosci Lett. 2007;429:136–41.
Banerjee M, Sasse VA, Wang Y, Maulik M, Kar S. Increased levels and activity of cathepsins B and D in kainate-induced toxicity. Neuroscience. 2015;284:360–73.
Wan Y, Feng B, You Y, Yu J, Xu C, Dai H, et al. Microglial displacement of GABAergic synapses is a protective event during complex febrile seizures. Cell Rep. 2020;33:108346.
Devinsky O, Vezzani A, Najjar S, De Lanerolle NC, Rogawski MA. Glia and epilepsy: excitability and inflammation. Trends Neurosci. 2013;36:174–84.
Hiragi T, Ikegaya Y, Koyama R. Microglia after seizures and in epilepsy. Cells. 2018;7:26.
Di Nunzio M, Di Sapia R, Sorrentino D, Kebede V, Cerovic M, Gullotta GS, et al. Microglia proliferation plays distinct roles in acquired epilepsy depending on disease stages. Epilepsia. 2021;62:1931–45.
Andoh M, Ikegaya Y, Koyama R. Synaptic pruning by microglia in epilepsy. J Clin Med. 2019;8:2170.
Rosciszewski G, Cadena V, Auzmendi J, Cieri MB, Lukin J, Rossi AR, et al. Detrimental effects of HMGB-1 require microglial-astroglial interaction: implications for the status epilepticus-induced neuroinflammation. Front Cell Neurosci. 2019;13:380.
Shi Y, Zhang L, Teng J, Miao W. HMGB1 mediates microglia activation via the TLR4/NF-κB pathway in coriaria lactone induced epilepsy. Mol Med Rep. 2018;17:5125–31.
Wang N, Mi X, Gao B, Gu J, Wang W, Zhang Y, et al. Minocycline inhibits brain inflammation and attenuates spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Neuroscience. 2015;287:144–56.
Acknowledgements
This project was supported by grants from the National Key R&D program of China (2021ZD0202803 and 2020YFA0803902), the National Natural Science Foundation of China (82022071), and the Natural Science Foundation of Zhejiang Province (LD22H310003).
Author information
Authors and Affiliations
Contributions
ZC, YW, and SBP designed the research. SJD, YYS, MQY, and XYQ conducted the experiments. SJD, YZ, and YW conducted the data analysis. YZ, JYSun, ZSL, JYShi, CLX, WSC, SY, SBP, MN, YW, and ZC provided technical guidance and contributed to the data discussion. SJD, YYS, YZ, and YW wrote the manuscript. YW and ZC supervised all aspects of the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dai, Sj., Shao, Yy., Zheng, Y. et al. Inflachromene attenuates seizure severity in mouse epilepsy models via inhibiting HMGB1 translocation. Acta Pharmacol Sin 44, 1737–1747 (2023). https://doi.org/10.1038/s41401-023-01087-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-023-01087-6