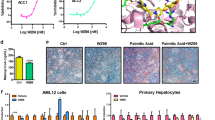
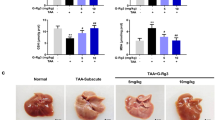
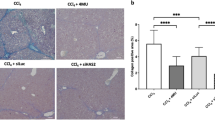
Abstract
UDP-glucose ceramide glucosyltransferase (UGCG) is the first key enzyme in glycosphingolipid (GSL) metabolism that produces glucosylceramide (GlcCer). Increased UGCG synthesis is associated with cell proliferation, invasion and multidrug resistance in human cancers. In this study we investigated the role of UGCG in the pathogenesis of hepatic fibrosis. We first found that UGCG was over-expressed in fibrotic livers and activated hepatic stellate cells (HSCs). In human HSC-LX2 cells, inhibition of UGCG with PDMP or knockdown of UGCG suppressed the expression of the biomarkers of HSC activation (α-SMA and collagen I). Furthermore, pretreatment with PDMP (40 μM) impaired lysosomal homeostasis and blocked the process of autophagy, leading to activation of retinoic acid signaling pathway and accumulation of lipid droplets. After exploring the structure and key catalytic residues of UGCG in the activation of HSCs, we conducted virtual screening, molecular interaction and molecular docking experiments, and demonstrated salvianolic acid B (SAB) from the traditional Chinese medicine Salvia miltiorrhiza as an UGCG inhibitor with an IC50 value of 159 μM. In CCl4-induced mouse liver fibrosis, intraperitoneal administration of SAB (30 mg · kg−1 · d−1, for 4 weeks) significantly alleviated hepatic fibrogenesis by inhibiting the activation of HSCs and collagen deposition. In addition, SAB displayed better anti-inflammatory effects in CCl4-induced liver fibrosis. These results suggest that UGCG may represent a therapeutic target for liver fibrosis; SAB could act as an inhibitor of UGCG, which is expected to be a candidate drug for the treatment of liver fibrosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Novo E, Parola M. The role of redox mechanisms in hepatic chronic wound healing and fibrogenesis. Fibrogenes Tissue Repair. 2012;5:S4.
Zhang CY, Yuan WG, He P, Lei JH, Wang CX. Liver fibrosis and hepatic stellate cells: etiology, pathological hallmarks and therapeutic targets. World J Gastroenterol. 2016;22:10512–22.
Kisseleva T, Brenner D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol. 2021;18:151–66.
Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397–411.
Ezhilarasan D, Sokal E, Najimi M. Hepatic fibrosis: It is time to go with hepatic stellate cell-specific therapeutic targets. Hepatobiliary Pancreat Dis Int. 2018;17:192–7.
Trivedi P, Wang S, Friedman SL. The power of plasticity-metabolic regulation of hepatic stellate cells. Cell Metab. 2021;33:242–57.
Mao YQ, Fan XM. Autophagy: A new therapeutic target for liver fibrosis. World J Hepatol. 2015;7:1982–6.
Gao J, Wei B, de Assuncao TM, Liu Z, Hu X, Ibrahim S, et al. Hepatic stellate cell autophagy inhibits extracellular vesicle release to attenuate liver fibrosis. J Hepatol. 2020;73:1144–54.
Thoen LF, Guimaraes EL, Dolle L, Mannaerts I, Najimi M, Sokal E, et al. A role for autophagy during hepatic stellate cell activation. J Hepatol. 2011;55:1353–60.
Hong Y, Li S, Wang J, Li Y. In vitro inhibition of hepatic stellate cell activation by the autophagy-related lipid droplet protein ATG2A. Sci Rep. 2018;8:9232.
Hernandez-Gea V, Hilscher M, Rozenfeld R, Lim MP, Nieto N, Werner S, et al. Endoplasmic reticulum stress induces fibrogenic activity in hepatic stellate cells through autophagy. J Hepatol. 2013;59:98–104.
Tuohetahuntila M, Molenaar MR, Spee B, Brouwers JF, Wubbolts R, Houweling M, et al. Lysosome-mediated degradation of a distinct pool of lipid droplets during hepatic stellate cell activation. J Biol Chem. 2017;292:12436–48.
Lee TF, Mak KM, Rackovsky O, Lin YL, Kwong AJ, Loke JC, et al. Downregulation of hepatic stellate cell activation by retinol and palmitate mediated by adipose differentiation-related protein (ADRP). J Cell Physiol. 2010;223:648–57.
Jing XY, Yang XF, Qing K, Ou-yang Y. Roles of the lipid metabolism in hepatic stellate cells activation. Chin Med Sci J. 2013;28:233–6.
Schomel N, Gruber L, Alexopoulos SJ, Trautmann S, Olzomer EM, Byrne FL, et al. UGCG overexpression leads to increased glycolysis and increased oxidative phosphorylation of breast cancer cells. Sci Rep. 2020;10:8182.
Schomel N, Hancock SE, Gruber L, Olzomer EM, Byrne FL, Shah D, et al. UGCG influences glutamine metabolism of breast cancer cells. Sci Rep. 2019;9:15665.
Schomel N, Geisslinger G, Wegner MS. Influence of glycosphingolipids on cancer cell energy metabolism. Prog Lipid Res. 2020;79:101050.
Li Z, Zhang L, Liu D, Wang C. Ceramide glycosylation and related enzymes in cancer signaling and therapy. Biomed Pharmacother. 2021;139:111565.
Zhuo D, Li X, Guan F. Biological roles of aberrantly fxpressed glycosphingolipids and related enzymes in human cancer development and progression. Front Physiol. 2018;9:466.
Guri Y, Colombi M, Dazert E, Hindupur SK, Roszik J, Moes S, et al. mTORC2 promotes tumorigenesis via lipid synthesis. Cancer Cell. 2017;32:807–23.e12.
Jennemann R, Federico G, Mathow D, Rabionet M, Rampoldi F, Popovic ZV, et al. Inhibition of hepatocellular carcinoma growth by blockade of glycosphingolipid synthesis. Oncotarget. 2017;8:109201–16.
Bangen JM, Hammerich L, Sonntag R, Baues M, Haas U, Lambertz D, et al. Targeting CCl4-induced liver fibrosis by RNA interference-mediated inhibition of cyclin E1 in mice. Hepatology. 2017;66:1242–57.
Tao XM, Li D, Zhang C, Wen GH, Wu C, Xu YY, et al. Salvianolic acid B protects against acute and chronic liver injury by inhibiting Smad2C/L phosphorylation. Exp Ther Med. 2021;21:341.
Modak RV, Zaiss DM. Isolation and culture of murine hepatic stellate cells. Bio Protoc. 2019;9:e3422.
Fenderson BA, Ostrander GK, Hausken Z, Radin NS, Hakomori S. A ceramide analogue (PDMP) inhibits glycolipid synthesis in fish embryos. Exp Cell Res. 1992;198:362–6.
Zhong B, Liu M, Bai C, Ruan Y, Wang Y, Qiu L, et al. Caspase-8 induces lysosome-associated cell death in cancer cells. Mol Ther. 2020;28:1078–91.
Li Z, Chiang YP, He M, Worgall TS, Zhou H, Jiang XC. Liver sphingomyelin synthase 1 deficiency causes steatosis, steatohepatitis, fibrosis, and tumorigenesis: An effect of glucosylceramide accumulation. iScience. 2021;24:103449.
Iredale JP, Thompson A, Henderson NC. Extracellular matrix degradation in liver fibrosis: Biochemistry and regulation. Biochim Biophys Acta. 2013;1832:876–83.
Ortiz C, Schierwagen R, Schaefer L, Klein S, Trepat X, Trebicka J. Extracellular matrix remodeling in chronic liver disease. Curr Tissue Microenviron Rep. 2021;2:41–52.
Roderfeld M. Matrix metalloproteinase functions in hepatic injury and fibrosis. Matrix Biol. 2018;68-69:452–62.
Zhang J, Jiang N, Ping J, Xu L. TGF-β1-induced autophagy activates hepatic stellate cells via the ERK and JNK signaling pathways. Int J Mol Med. 2021;47:256–66.
Deng J, Huang Q, Wang Y, Shen P, Guan F, Li J, et al. Hypoxia-inducible factor-1alpha regulates autophagy to activate hepatic stellate cells. Biochem Biophys Res Commun. 2014;454:328–34.
Chen M, Liu J, Yang W, Ling W. Lipopolysaccharide mediates hepatic stellate cell activation by regulating autophagy and retinoic acid signaling. Autophagy. 2017;13:1813–27.
Myerowitz R, Puertollano R, Raben N. Impaired autophagy: The collateral damage of lysosomal storage disorders. EBioMedicine. 2021;63:103166.
Koike K, Berdyshev EV, Mikosz AM, Bronova IA, Bronoff AS, Jung JP, et al. Role of glucosylceramide in lung endothelial cell fate and emphysema. Am J Respir Crit Care Med. 2019;200:1113–25.
Bodas M, Min T, Vij N. Lactosylceramide-accumulation in lipid-rafts mediate aberrant-autophagy, inflammation and apoptosis in cigarette smoke induced emphysema. Apoptosis. 2015;20:725–39.
Hartwig P, Hoglinger D. The glucosylceramide synthase inhibitor PDMP causes lysosomal lipid accumulation and mTOR inactivation. Int J Mol Sci. 2021;22:7065.
Feng J, Chen K, Xia Y, Wu L, Li J, Li S, et al. Salidroside ameliorates autophagy and activation of hepatic stellate cells in mice via NF-kappaB and TGF-β1/Smad3 pathways. Drug Des Devel Ther. 2018;12:1837–53.
Ji J, Yu Q, Dai W, Wu L, Feng J, Zheng Y, et al. Apigenin alleviates liver fibrosis by inhibiting hepatic stellate cell activation and autophagy via TGF-β1/Smad3 and p38/PPARalpha Pathways. PPAR Res. 2021;2021:6651839.
Meng D, Li Z, Wang G, Ling L, Wu Y, Zhang C. Carvedilol attenuates liver fibrosis by suppressing autophagy and promoting apoptosis in hepatic stellate cells. Biomed Pharmacother. 2018;108:1617–27.
Ichikawa S, Sakiyama H, Suzuki G, Hidari KI, Hirabayashi Y. Expression cloning of a cDNA for human ceramide glucosyltransferase that catalyzes the first glycosylation step of glycosphingolipid synthesis. Proc Natl Acad Sci USA. 1996;93:4638–43.
Ichikawa S, Ozawa K, Hirabayashi Y. Molecular cloning and expression of mouse ceramide glucosyltransferase. Biochem Mol Biol Int. 1998;44:1193–202.
Ramos-Tovar E, Muriel P. Molecular mechanisms that link oxidative stress, inflammation, and fibrosis in the liver. Antioxidants (Basel). 2020;9:1279.
Lu C, Zou Y, Liu Y, Niu Y. Rosmarinic acid counteracts activation of hepatic stellate cells via inhibiting the ROS-dependent MMP-2 activity: Involvement of Nrf2 antioxidant system. Toxicol Appl Pharmacol. 2017;318:69–78.
Shibayama K, Ohsuka S, Sato K, Yokoyama K, Horii T, Ohta M. Four critical aspartic acid residues potentially involved in the catalytic mechanism of Escherichia coli K-12 WaaR. FEMS Microbiol Lett. 1999;174:105–9.
Li D, Fournel-Gigleux S, Barre L, Mulliert G, Netter P, Magdalou J, et al. Identification of aspartic acid and histidine residues mediating the reaction mechanism and the substrate specificity of the human UDP-glucuronosyltransferases 1A. J Biol Chem. 2007;282:36514–24.
Xiong Y, Patana AS, Miley MJ, Zielinska AK, Bratton SM, Miller GP, et al. The first aspartic acid of the DQxD motif for human UDP-glucuronosyltransferase 1A10 interacts with UDP-glucuronic acid during catalysis. Drug Metab Dispos. 2008;36:517–22.
Campesato L, Marforio TD, Giacinto P, Calvaresi M, Bottoni A. A full QM computational study of the catalytic mechanism of alpha-1,4-glucan lyases. Chemphyschem. 2018;19:1514–21.
Wu K, Marks DL, Watanabe R, Paul P, Rajan N, Pagano RE. Histidine-193 of rat glucosylceramide synthase resides in a UDP-glucose- and inhibitor (D-threo-1-phenyl-2-decanoylamino-3-morpholinopropan-1-ol)-binding region: a biochemical and mutational study. Biochem J. 1999;341:395–400.
Tsai MK, Lin YL, Huang YT. Effects of salvianolic acids on oxidative stress and hepatic fibrosis in rats. Toxicol Appl Pharmacol. 2010;242:155–64.
Wang R, Song F, Li S, Wu B, Gu Y, Yuan Y. Salvianolic acid A attenuates CCl4-induced liver fibrosis by regulating the PI3K/AKT/mTOR, Bcl-2/Bax and caspase-3/cleaved caspase-3 signaling pathways. Drug Des Devel Ther. 2019;13:1889–900.
Shi MJ, Yan XL, Dong BS, Yang WN, Su SB, Zhang H. A network pharmacology approach to investigating the mechanism of Tanshinone IIA for the treatment of liver fibrosis. J Ethnopharmacol. 2020;253:112689.
Lan Y, Yan R, Shan W, Chu J, Sun R, Wang R, et al. Salvianic acid A alleviates chronic alcoholic liver disease by inhibiting HMGB1 translocation via down-regulating BRD4. J Cell Mol Med. 2020;24:8518–31.
Xiao Z, Liu W, Mu YP, Zhang H, Wang XN, Zhao CQ, et al. Pharmacological effects of salvianolic acid B against oxidative damage. Front Pharmacol. 2020;11:572373.
Li CL, Liu B, Wang ZY, Xie F, Qiao W, Cheng J, et al. Salvianolic acid B improves myocardial function in diabetic cardiomyopathy by suppressing IGFBP3. J Mol Cell Cardiol. 2020;139:98–112.
Tao YY, Wang QL, Shen L, Fu WW, Liu CH. Salvianolic acid B inhibits hepatic stellate cell activation through transforming growth factor beta-1 signal transduction pathway in vivo and in vitro. Exp Biol Med (Maywood). 2013;238:1284–96.
Tian S, Chen M, Wang B, Han Y, Shang H, Chen J. Salvianolic acid B blocks hepatic stellate cell activation via FGF19/FGFR4 signaling. Ann Hepatol. 2021;20:100259.
Zhang W, Ping J, Zhou Y, Chen G, Xu L. Salvianolic acid B inhibits activation of human primary hepatic stellate cells through downregulation of the myocyte enhancer factor 2 signaling pathway. Front Pharmacol. 2019;10:322.
Acknowledgements
We are truly grateful to the oncomine, GEO and alphafold working groups for generously sharing their data. This work was supported by the National Natural Science Foundation of China (Grant No. 81930114), Key-Area Research and Development Program of Guangdong Province (Grant No. 2020B1111100004), the 2020 Guangdong Provincial Science and Technology Innovation Strategy Special Fund (Guangdong-Hong Kong-Macau Joint Laboratory, Grant No. 2020B1212030006).
Author information
Authors and Affiliations
Contributions
ZBL and LJ performed the experiments; SGW, ZQL, and CYW designed the project and revised the paper; ZBL, LJ, JDN, YHX, FL, WML performed the informatics analysis and experiments; ZBL and CYW wrote the paper. All authors read and approved the final paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Zb., Jiang, L., Ni, Jd. et al. Salvianolic acid B suppresses hepatic fibrosis by inhibiting ceramide glucosyltransferase in hepatic stellate cells. Acta Pharmacol Sin 44, 1191–1205 (2023). https://doi.org/10.1038/s41401-022-01044-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-022-01044-9
Keywords
This article is cited by
-
UGCG modulates heart hypertrophy through B4GalT5-mediated mitochondrial oxidative stress and the ERK signaling pathway
Cellular & Molecular Biology Letters (2023)