Abstract
Immunotherapy that activates immune systems for combating cancer has yielded considerable clinical benefits recently. However, the immunosuppressive tumor microenvironment (ITME) is a major hurdle to immunotherapy as it supports tumor to evade immune surveillance. Reversing ITME facilitates the recruitment and activation of antitumor immune cells, thereby promoting immunotherapy. Our group has developed various nanosized drug delivery systems (NDDSs) to modulate ITME with enhanced efficacy and safety. In the review we introduce the ITME-remodeling strategies for improving immunotherapy based on NDDSs including triggering tumor cells to undergo immunogenetic cell death (ICD), applying tumor vaccine, and directly regulating intratumoral immune components (immune cells or cytokines). In order to guide the design of NDDSs for amplified effects of antitumor immunotherapy, the contributions and future directions of this field are also discussed.
Similar content being viewed by others
Introduction
Immunotherapy, which activates immune systems to combat cancer, has yielded considerable clinical benefits recently [1]. Antitumor immunotherapy can be typically divided into two categories based on the mechanism: (i) immune-enhancing therapies that reinforce immune responses against tumors, such as cytokines, vaccines, and adoptive cell therapy, and (ii) immune-normalizing therapies that repair the defects of systemic antitumor immunity, such as immune checkpoint blockade (ICB) [2]. Despite wide application in the clinic, how to obtain long-lasting responses in the majority of patients suffering cancer remains an unsolved problem for cancer immunotherapy [3].
Severe immune-related side effects (irSEs) and a low response rate hinder the progress of immunotherapy [4, 5]. The irSEs (e.g., myocarditis and pneumonia) result from nonspecific activation of the immune system due to the extratumoral distribution of the drug after systemic administration. Primary and acquired resistance in tumors results in the ineffectiveness of immunotherapy [6]. For instance, the objective response rates of programmed death 1 (PD-1) protein or its ligand (PD-L1) inhibitors against certain cancers (e.g., pancreatic cancer and glioblastoma) are lower than 30% [7]. The immunosuppressive tumor microenvironment (ITME) is a major hurdle to immunotherapy, as it supports tumor evasion of immune surveillance [8]. Therefore, developing strategies to reverse the ITME is necessary for improving cancer immunotherapy [9, 10].
Nanosized drug delivery systems (NDDSs) improve the safety and efficacy of cancer immunotherapy because of their excellent pharmacokinetic and biodistribution profiles, including prolonged blood half-life, high intratumoral accumulation, and deep tumor penetration capacity [11,12,13]. They can also be designed as multidrug delivery platforms for combined therapy, which is beneficial for immunotherapy since ITME is an intricate network that requires modulation from multiple aspects [14,15,16].
Recently, our group reported a series of NDDSs for remodeling the ITME and enhancing cancer immunotherapy. In this review, we briefly introduce the composition of ITME and traditional treatments against ITME. Then, we will elaborate our endeavors in reversing the ITME to improve immunotherapy by manipulating NDDSs based on different strategies. Finally, the contributions and prospects of this field will be discussed.
Immunosuppressive tumor microenvironment
Characterization and composition
The tumor microenvironment (TME) is highly heterogeneous among tumors [17]. Based on the type, density and location of immune cells within the tumor site, the tumor immune microenvironment can be broadly classified into two categories: hot and cold tumors [18]. Immunologically hot tumors, with high infiltration of cytotoxic T lymphocytes (CTLs) and activation of the PD-1/PD-L1 signaling pathway, are responsive to immunotherapy [19]. Unfortunately, many tumors insensitive to immunotherapy are usually cold tumors with ITME, which are difficult to eradicate and associated with poor prognosis [20].
Multiple and complex factors contribute to the ITME. The low mutational burden and poor immunogenicity of tumors prevent recognition by the immune system [21, 22]. Various immunosuppressive cells and cytokines impede antitumor immune responses through different signaling pathways [23]. The extracellular matrix and chemokines of tumors block the penetration of antitumor immune cells [24, 25]. Both the physicochemical properties of the tumor, such as hypoxia [26] and weak acidity [27], and abnormal metabolic activities, such as the accumulation of adenosine [28] and increased metabolism of L-arginine [29], facilitate the immune escape of tumors.
Traditional immunomodulatory methods
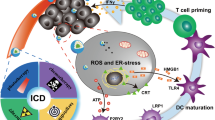
A range of approaches have been developed based on the same goal: to enhance antitumor immunity. Since ITME is an outcome of the parallel occurrence of multiplex protumor mechanisms, combination therapies have the potential to harvest clinical benefits [30]. Immunogenetic cell death (ICD), induced by radiotherapy, phototherapy, and certain chemotherapeutic drugs, such as oxaliplatin, is a type of cell death accompanied by the release of damage-associated molecular patterns (DAMPs) [31]. Tumor cells undergoing ICD will mature dendritic cells (DCs) to cross-present tumor-associated antigens (TAAs) to CD4+ and CD8+ T cells and thus activate an adaptive immune response. In addition, released DAMPs promote phagocytosis and boost the innate immune response. Tumor vaccines or immune adjuvants can also transform cold tumors into hot tumors [32]. Targeted therapies to increase tumor immunogenicity [33], cytokine therapies to activate T cells [34], and oncolytic viruses to release TAAs [35] are potential strategies to propel immunotherapy as well.
However, traditional combination therapies also face the problem of undesired side effects and unsatisfactory efficiency. The nonspecific distribution of therapeutic agents after systemic administration causes damage to healthy organs and reduces the concentration and effects of the drug at the tumor sites. In addition, other therapies in combination with immunotherapy may affect the immune system, which limits the synergetic effects [36]. For instance, lymphodepletion and impact on tertiary lymphoid structures by chemotherapy may hinder the outcome of immunotherapy. The clinical responses of radioimmunotherapy combinations are contradictory since radiation kills tumor cells as well as immune effector cells. Therefore, strategies for targeted drug delivery are necessary to amplify the effects and reduce the toxicity of each component utilized in combination therapy.
NDDSs reversing ITME for enhancing cancer immunotherapy
NDDSs, with higher efficiency and safety than traditional treatments, can deliver immunomodulatory drugs specifically to tumor sites through passive or/and active targeting [37]. They can also be endowed with TME sensitivity to release payloads specifically at tumor sites, which further lessens adverse toxicities [38]. Moreover, multidrug-loaded nanoplatforms provide options for exerting the synergistic effects of combined treatments [39]. In this section, we will mainly introduce the relevant works with the categorization of the strategies to reverse ITME (Fig. 1 and Table 1).
Modulating the ITME based on NDDSs through promoting antigen release of tumor cells, maturation of dendritic cells (DCs), activation of cytotoxic T lymphocytes (CTLs), and tumor-killing effects of CTLs. DAMP damage-associated molecular pattern, TAA tumor-associated antigen, ICD immunogenetic cell death, TME tumor microenvironment, TAM tumor-associated macrophage, ROS reactive oxygen species, PD-1/PD-L1 programmed death 1/PD-1 ligand, IDO indoleamine 2,3-dioxygenase.
Combining ICD-inducing therapy with immunotherapy
ICD-inducing treatments provide an immune-activation environment for immunotherapy. Additionally, immunotherapy, including ICB and indoleamine 2,3-dioxygenase (IDO) inhibition, compensates for the upregulation of PD-L1 and IDO caused by IFN-γ, which is secreted by ICD-activated CTLs [40]. NDDSs can deliver targeted toxic ICD-inducing agents, including chemotherapeutic agents, radiotherapy sensitizers, and photosensitizers, to tumor sites, thus minimizing adverse effects and improving therapeutic outcomes [41, 42].
ICD induced by chemotherapy
The combination of chemotherapy and immunotherapy is an encouraging strategy because of the ICD-inducing ability of some chemotherapeutics. To improve the targeting and accessibility of ICD inducers to tumor cells, Li et al. fabricated a bioinspired lipoprotein system containing the legumain-sensitive melittin prodrug, the pH-sensitive phospholipid, and the nitroreductase-sensitive oxaliplatin [43]. After administration, the release of melittin by high-level legumain in tumors promoted the intratumoral permeation of the nanoplatform, and then, the size-enlargement of the nanoplatform after internalization as a response to acidity released oxaliplatin prodrug. Finally, the stimulation of oxaliplatin by nitroreductase and reductive environments induced ICD and elicited antitumor responses (Fig. 2a). Treatment with the nanoplatform increased the proportion of intratumoural CTLs and mature DCs by 1.76-fold and 3.57-fold, respectively, compared with free oxaliplatin treatment, indicating the importance of the delivery strategy. The combination of immune-activating treatment and ICB prolonged the survival of tumor-bearing mice compared with single therapy, demonstrating that preregulating the TME facilitated the effects of immunotherapy. A nanovesicle constructed by fusing artificial liposomes with tumor-derived nanovesicles was utilized for the targeted delivery of doxorubicin, which improved the immunogenicity of tumors and improved the therapeutic efficacy of ICB [44].
a Schematic illustration of the cancer-accessing tumor-activated size-enlargeable bioinspired lipoprotein system (TA-OBL) to boost antitumor immune responses and synergize with ICB-mediated immunotherapy. b Schematic illustration of the procedures for the evaluation of the activity of sHDL in inducing DC maturation and the proposed mechanism of action. c Treatment-induced extracellular release of ATP and HMGB1 from Hepa1-6 cells treated with different sHDLs. All of the experiments were performed in triplicate, and the data are presented as the mean ± SD (n = 3). *P < 0.05; **P < 0.01; ***P < 0.001. d Schematic illustration of light-inducible nanocargoes (LINC) for improved drug delivery and chemoimmunotherapy by eliciting tumor immunogenicity and overcoming the immunosuppressive tumor microenvironment. e Tumor growth curves in 4T1 tumor-bearing mice after the indicated treatments (n = 6). Data are the mean ± SD. Statistical significance was calculated by one-way ANOVA. a Reproduced from Li et al. [43]. Copyright (2020) John Wiley & Sons. b, c Reproduced from Wang et al. [54]. Copyright (2019) American Chemical Society. d, e Reproduced from Feng et al. [59]. Copyright (2019) John Wiley & Sons.
In addition to sequential delivery, codelivery of ICD inducers and immunotherapeutic agents by NDDSs is also a potential strategy. The tryptophan catabolic enzyme IDO inhibits immune effector cells and promotes immunosuppressive cells [45]. A nanoparticle containing oxaliplatin prodrugs and the IDO inhibitor NLG919 [46] and a biomimetic micelle/monocyte delivery system containing docetaxel, NLG919 and a PD-1/PD-L1 inhibitor [47] were constructed to enhance the efficiency of the drugs and amplify the synergetic effects. These NDDSs provide robust platforms for chemoimmunotherapy to mature DCs and powerfully activate CTLs.
ICD induced by phototherapy
Phototherapy, including photodynamic therapy (PDT) and photothermal therapy (PTT), is capable of inducing ICD by generating reactive oxygen species (ROS) or local hyperthermia, respectively [41]. Given the high expression of matrix metalloproteinase 2 (MMP-2) in the TME, Wang and coworkers encapsulated an anti-PD-L1 antibody and a photosensitizer into MMP-2-responsive nanoparticles to precisely release drugs at the tumor site. Photosensitizer-mediated PDT under a near-infrared (NIR) laser sensitized the tumors to ICB [48]. Consequently, the tumor inhibition rate of mice treated with the nanoplatform and irradiation was 73.2%, which was only 39.8% of the free antibody-treated group. Similarly, a pH-sensitive nanoplatform loaded with a photosensitizer and a PD-L1 siRNA was constructed to overcome the immunological tolerance of tumors [49]. TME-activated nanoplatforms codelivering photosensitizers and IDO inhibitors [50, 51] or Toll-like receptor 3 (TLR3) agonists [52] also amplify antitumor immunity. These works based on NDDSs exemplify the flexibility of the combination of PDT and immunotherapy, providing valuable cases for subsequent combination therapies. Furthermore, oxygen supplementation should be implemented to improve treatment outcomes, as the hypoxia of tumors limits the efficacy of PDT [53].
PTT allows photothermal imaging and enhances drug penetration while ablating tumors. Wang et al. demonstrated a nanoplatform based on synthetic high-density lipoprotein (sHDL) containing a photothermal agent and an immune adjuvant [54]. The nanoplatform triggered a more than fivefold higher release of adenosine-5′-triphosphate (ATP) and high mobility group Box 1 (HMGB1) (two DAMPs) than phosphate buffered saline (PBS) (Fig. 2b, c). Additionally, an all-in-one nanoparticle containing sorafenib, Prussian blue as an oxygen-generating catalyst and cyanine 5.5 as a photothermal agent was developed to mediate PTT and relieve hypoxia [55]. The PTT-mediated nanoplatforms demonstrated the ability to synergize with anti-PD-1/PD-L1 therapies to inhibit primary and metastatic tumors.
ICD induced by radiotherapy
Radiotherapy, as a local therapy, can exert an abscopal effect by ionizing ROS and thus triggering ICD [56]. A series of NDDSs designed to targeted deliver radiosensitizers and immunotherapeutic agents have been reported by a number of research groups, eliminating tumors by boosting immune responses effectively [57]. For instance, Wang et al. reported a nanosheet constructed with a radiosensitizer and an inhibitor of the pentose phosphate pathway to amplify the oxidative stress and DNA damage induced by radiotherapy [58]. Effective radiotherapy induced ICD and primed CD8+-T-cell-dependent antitumor immune responses, providing a hot tumor immune environment for anti-PD-L1 therapy.
ICD induced by combinational therapies
Combining chemotherapy with phototherapy is capable of complementing each other to induce a stronger ICD. A light-activatable nanoplatform containing a photosensitizer, oxaliplatin and NLG919 was fabricated to achieve spatiotemporally controlled drug accumulation and deep tumor penetration [59]. The first laser irradiation shed the polyethylene glycol block of the prodrug, enhancing the distribution of the drug in the tumor to 3.5-fold that of irradiation-free treatment. The second irradiation performed PDT, inducing ICD of tumor cells along with oxaliplatin (Fig. 2d). Additionally, NLG919 attenuated immunosuppression of the TME. The synergetic therapy eradicated 67% of the 4T1 tumors, while the growth of the tumors treated with monotherapy lost control (Fig. 2e). Moreover, an enzyme- and ROS-activatable nanovesicle, able to trigger ICD by chemotherapy and PDT, was constructed to augment antitumor immune responses with the assistance of anti-CD47 therapy promoting phagocytosis of tumor cells by DCs and macrophages [60]. The works mentioned above provide a paradigm for exploiting the synergistic effects of polytherapy, demonstrating the advantages of multidrug-loaded NDDSs.
Applying tumor vaccines
Tumor vaccines, classified as proteins or peptides of cancer antigens, nucleic acids, and cell-based tumor antigens, initiate specific antitumor immune responses [61]. However, the clinical translation of tumor vaccines has been difficult [62]. Optimizing the delivery strategy may benefit tumor vaccines. Various nanovaccines have been developed because of their improved antigen stability, bioavailability, and lymphatic drainage [63].
Autologous tumor cell-based vaccines (ATVs) enable personalized immunotherapy. To amplify the activation of CTLs by ATVs, Wang et al. encapsulated tumor cells loaded with the PD-L1 inhibitor JQ1 and the photothermal agent indocyanine green (ICG) into a hydrogel, which promoted tumor penetration and retention of the vaccines (Fig. 3a) [64]. Laser irradiation triggered the release of JQ1 and vaccines. The levels of IFN-γ, tumor necrosis factor α (TNF-α), and interleukin 6 (IL-6) were higher in mice treated with vaccine-loaded hydrogel and irradiation than in mice treated with vaccine-free or irradiation-free treatment (Fig. 3b–d). The combinational treatment achieved a 62% maturation rate of DCs in the tumor-draining lymph nodes, which was 3.1-fold that of the treatment without vaccine loading, contributing to the inhibition of tumor relapse (Fig. 3e, f). Another ATV-containing hydrogel system triggered by PDT remodeled the immune compositions as well, providing a viable strategy for personalized immunotherapy to eradicate postoperative tumors [65].
a Fabrication process of the personalized cancer vaccine (PVAX). (b-d) Serum concentrations of TNF-α (b), IFN-γ (c), and IL-6 (d) examined at the desired time points post treatment. (n = 3). e The frequency of mature DCs (CD11c+CD80+CD86+) in draining LNs of BALB/c mice upon different treatments. Data represent the mean ± SD (n = 3). f Tumor-free percentages of BALB/c mice rechallenged with distant tumors. Reproduced from Wang et al. [64]. Copyright (2018) Springer Nature.
Tumor neoantigen vaccines are widely used for immunotherapy. To improve the antigen-presenting ability of DCs, Zhou and coworkers designed a pH-sensitive nanoplatform to codeliver a neoantigen and a stimulator of interferon genes (STING) agonist [66]. The nanovaccine induced DC maturation and antigen presentation synergized with STING activation, achieving an 80% tumor inhibition rate in melanoma tumor-bearing mice and decreasing the tumor occurrence rate. The combination with an anti-PD-L1 antibody further restrained tumor immune escape.
Delivery of tumor antigens to lymph nodes, internalization by DCs, and induction of the costimulatory signaling of DCs are important steps in the activation of antitumor CTLs by nanovaccines [67]. To avoid the reduced efficacy during these steps, Xiao et al. proposed an artificial antigen-presenting cell (aAPC) to directly stimulate T cells [68]. Glycoengineering and tumor antigen stimulation endowed DC membranes with anti-CD3 antibody and antigen-major histocompatibility complex I (MHC I). Imiquimod-containing nanoparticles encapsulated by the DC membrane targeted and retained in lymph nodes and stimulated T cells via anti-CD3 antibodies, CD28 naturally expressed on DCs, and antigen-MHC I complexes. In addition, imiquimod enabled nanoparticles to reprogram macrophages toward the pro-inflammatory M1-type after phagocytosis. Additionally, a synergistic mobilization of CTLs by the combination of tumor lysate-loaded DCs and nanovaccines containing neoantigens was demonstrated [69]. A nanovesicle based on a genetically engineered DC membrane with lymphatic system homing and antigen self-presentation ability could also stimulate antitumor immune responses and reverse immune tolerance [70]. These works present new insights for high-performance immunotherapy by optimizing the access and stimulation of T cells by tumor vaccines.
Regulating immune cells or cytokines directly
High infiltration of immunosuppressive cells, such as myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages (TAMs), and low expression of antitumor cytokines are essential causes of ITME. Therefore, we designed NDDS-based strategies directly regulating immunosuppressive cells and cytokines.
Since sHDL could target bone marrow-derived cells (MDCs), a sHDL nanoparticle was utilized to encapsulate vadimezan prodrug and gemcitabine [71]. Vadimezan promoted the differentiation of MDCs to DCs, while gemcitabine significantly upregulated the intratumoral proportion of M1 macrophages by killing M2 macrophages and inducing monocyte differentiation. The nanoparticles increased the intratumoral M1/M2 ratio by 20.5-fold compared with that in the PBS-treated group, reversing immunosuppression and inducing long-term immune memory to combat tumors. Other nanoplatforms for re-education of TAMs and suppression of MDSCs were constructed as reported, providing adequate references for establishing approaches aimed at rousing intrinsic and adaptive antitumor immune responses [72, 73].
Cytokine therapy was the first cancer immunotherapy approved by the Food and Drug Administration (FDA) [74]. However, the narrow therapeutic window limits its development, and designing strategies for safe and effective cytokine regulation is urgent [75]. IFN-γ deficiency leads to CTL inactivation and regulatory T-cell proliferation, but excess IFN-γ in turn upregulates PD-L1 expression. To resolve this paradox, Zhai and coworkers designed an intelligent NDDS named OPEN covered by PD-L1-overexpressing T-cell membranes and loaded with the IFN-γ inducer ORY-1001 (Fig. 4a) [76]. The biomimetic nanoplatform targeted tumor cells via the PD-1/PD-L1 interaction and released ORY-1001 after endocytosis to induce IFN-γ expression via epigenetic regulation. Consequently, PD-L1 on the tumor cells was upregulated, which improved the uptake of the nanoplatform. In addition, PD-1 on the nanoplatform blocked the upregulation of PD-L1, thereby preventing immune escape (Fig. 4b, c). The treatment attenuated tumor growth with a 73% inhibition rate and prolonged the median survival time in tumor-bearing mice (Fig. 4d). ITME is a complex system, and regulating some components inevitably promotes immunosuppression through negative feedback regulation. This strategy provides new insights to resolve this contradiction by turning enemies (upregulation of PD-L1) into friends (increased uptake of the drug). Direct delivery of cytokines based on nanoplatforms to tumor sites could reduce systemic toxicity and reverse the ITME [77, 78].
a Preparation of the ORY-1001-loaded and PD1-overexpressing T lymphocyte membrane-decorated epigenetic nanoinducer (OPEN). M70, macrolittin 70. b OPEN is expected to recognize and enter PDL1-expressing cells and release ORY-1001 with the help of M70. ORY-1001 can upregulate the expression of IFNs, MHC-I and PDL1. Upregulated PDL1 is neutralized by subsequent OPEN. ERV, endogenous retrovirus. c After intravenous injection and tumor accumulation, OPEN is able to enhance the recruitment, proliferation and activity of CTLs in the tumor. TCR, T-cell receptor; GzmB, granzyme. d Survival curves of mice with 4T1, CT26 or B16-F10 tumors (n = 6 biologically independent animals for the 4T1 model and n = 7 biologically independent animals for the CT26 and B16-F10 models) receiving the indicated treatments. Statistical analysis was performed using two-way ANOVA and Tukey’s tests for tumor growth data and log-rank tests for survival data. Reproduced from Zhai et al. [76]. Copyright (2021) Springer Nature.
Conclusions and perspectives
NDDS-based ITME modulation, including ICD induction, tumor vaccination, and direct regulation of immune cells or cytokines, improves immunotherapy by increasing tumor immunogenicity, maturing DCs and activating CTLs, secreting antitumor cytokines, and promoting the tumor-killing effects of CTLs and phagocytes. Since immunotherapeutic drugs target different organs, cells and even subcellular sites, focusing the drug on the target site can increase the therapeutic window. Nanoplatforms equip immunomodulatory agents with spatiotemporally controllable release profiles to target specific organs (e.g., tumors or lymph nodes) and cells. ITME modulators and immunotherapeutic agents can be coencapsulated into nanocarriers with TME sensitivity and/or active targeting ability to improve their accessibility to target organs. They can also be constructed as prodrugs and self-assemble to form NDDSs with targeting ability. Additionally, delivery vehicles such as hydrogels are able to extend the retention time of drugs in the tumor and achieve long-lasting modulation of the ITME. Nanoplatforms for multidrug delivery can also facilitate multiple processes in the antitumor immune response cycle, acting synergistically to further enhance the effectiveness of immunotherapy [79, 80]. It is preferable to choose drugs that play roles at different steps of the antitumor immune cycle or target different immune components for combination to avoid redundancy of action.
However, a number of preclinical studies have failed in clinical translation due to the differences between animal tumor models and humans, difficulties in the production and quality control of nanosystems, and safety issues arising from the unclear in vivo behavior of NDDS components. Moreover, the modulation strength of NDDSs on a highly coordinated immune system is limited, as tumors are able to develop drug resistance through negative feedback regulation. The multilayered effects of the various components of NDDSs on the immune system may cause potential antagonistic effects and limit efficacy. Therefore, more studies are needed for bench-to-bedside translation. First, utilizing materials approved by the FDA or developing biosafe and biodegradable carrier materials is necessary, and their interaction with the immune system should be investigated. Specifically, the interaction at the molecular level between delivery systems and their target cells should be focused on since targeting different cellular receptors may produce different effects. Second, developing feasible NDDS preparation methods and reducing components, such as designing carrier-free NDDSs, are beneficial for industrial production. Third, multidrug combinations for immunotherapy require careful dose adjustment to maximize their synergistic effects and reduce toxicity. The dosing interval and drug-release profiles (slow release or immediate release) of NDDSs also need to be optimized to improve the therapeutic index. In addition, optimization of animal models, such as in situ tumor models, also benefits the clinical translation of NDDSs. With the rapid development of nanotechnology and tumor immunology, we believe that the use of NDDSs to modulate the ITME will facilitate the development of cancer immunotherapy and yield clinical benefits in the future.
References
Khalil DN, Smith EL, Brentjens RJ, Wolchok JD. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol. 2016;13:273–90.
Sanmamed MF, Chen L. A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell. 2018;175:313–26.
Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16:275–87.
June CH, Warshauer JT, Bluestone JA. Is autoimmunity the achilles’ heel of cancer immunotherapy? Nat Med. 2017;23:540–7.
Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158–68.
Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168:707–23.
Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med. 2017;377:2500–1.
Tormoen GW, Crittenden MR, Gough MJ. Role of the immunosuppressive microenvironment in immunotherapy. Adv Radiat Oncol. 2018;3:520–6.
O’Donnell JS, Teng MWL, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol. 2019;16:151–67.
Phuengkham H, Ren L, Shin IW, Lim YT. Nanoengineered immune niches for reprogramming the immunosuppressive tumor microenvironment and enhancing cancer immunotherapy. Adv Mater. 2019;31:e1803322.
Liu X, Wang D, Zhang P, Li Y. Recent advances in nanosized drug delivery systems for overcoming the barriers to anti-PD immunotherapy of cancer. Nano Today. 2019;29:100801.
Zhang P, Zhai Y, Cai Y, Zhao Y, Li Y. Nanomedicine-based immunotherapy for the treatment of cancer metastasis. Adv Mater. 2019;31:e1904156.
Goldberg MS. Improving cancer immunotherapy through nanotechnology. Nat Rev Cancer. 2019;19:587–602.
Nam J, Son S, Park KS, Zou W, Shea LD, Moon JJ. Cancer nanomedicine for combination cancer immunotherapy. Nat Rev Mater. 2019;4:398–414.
Qi FL, Wang MF, Li BZ, Lu ZF, Nie GJ, Li SP. Reversal of the immunosuppressive tumor microenvironment by nanoparticle-based activation of immune-associated cells. Acta Pharmacol Sin. 2020;41:895–901.
Sun B, Hyun H, Li LT, Wang AZ. Harnessing nanomedicine to overcome the immunosuppressive tumor microenvironment. Acta Pharmacol Sin. 2020;41:970–85.
Vitale I, Shema E, Loi S, Galluzzi L. Intratumoral heterogeneity in cancer progression and response to immunotherapy. Nat Med. 2021;27:212–24.
Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24:541–50.
Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18:197–218.
Bonaventura P, Shekarian T, Alcazer V, Valladeau-Guilemond J, Valsesia-Wittmann S, Amigorena S, et al. Cold tumors: a therapeutic challenge for immunotherapy. Front Immunol. 2019;10:168.
Samstein RM, Lee C-H, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51:202–6.
Westcott PMK, Sacks NJ, Schenkel JM, Ely ZA, Smith O, Hauck H, et al. Low neoantigen expression and poor T-cell priming underlie early immune escape in colorectal cancer. Nat Cancer. 2021;2:1071–85.
Li L, Yu R, Cai T, Chen Z, Lan M, Zou T, et al. Effects of immune cells and cytokines on inflammation and immunosuppression in the tumor microenvironment. Int Immunopharmacol. 2020;88:106939.
Huang J, Zhang L, Wan D, Zhou L, Zheng S, Lin S, et al. Extracellular matrix and its therapeutic potential for cancer treatment. Signal Transduct Target Ther. 2021;6:153.
Mao X, Xu J, Wang W, Liang C, Hua J, Liu J, et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: New findings and future perspectives. Mol Cancer. 2021;20:131.
You L, Wu W, Wang X, Fang L, Adam V, Nepovimova E, et al. The role of hypoxia-inducible factor 1 in tumor immune evasion. Med Res Rev. 2021;41:1622–43.
Wang JX, Choi SYC, Niu X, Kang N, Xue H, Killam J, et al. Lactic acid and an acidic tumor microenvironment suppress anticancer immunity. Int J Mol Sci. 2020;21:8363.
Leone RD, Emens LA. Targeting adenosine for cancer immunotherapy. J Immunother Cancer. 2018;6:57.
Geiger R, Rieckmann JC, Wolf T, Basso C, Feng Y, Fuhrer T, et al. L-arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell. 2016;167:1–14.
Xue G, Wang Z, Zheng N, Fang J, Mao C, Li X, et al. Elimination of acquired resistance to PD-1 blockade via the concurrent depletion of tumour cells and immunosuppressive cells. Nat Biomed Eng. 2021;5:1306–19.
Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12:860–75.
Paston SJ, Brentville VA, Symonds P, Durrant LG. Cancer vaccines, adjuvants, and delivery systems. Front Immunol. 2021;12:627932.
de Charette M, Marabelle A, Houot R. Turning tumour cells into antigen presenting cells: the next step to improve cancer immunotherapy? Eur J Cancer. 2016;68:134–47.
Berraondo P, Sanmamed MF, Ochoa MC, Etxeberria I, Aznar MA, Pérez-Gracia JL, et al. Cytokines in clinical cancer immunotherapy. Br J Cancer. 2019;120:6–15.
Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov. 2015;14:642–62.
Zhu S, Zhang T, Zheng L, Liu H, Song W, Liu D, et al. Combination strategies to maximize the benefits of cancer immunotherapy. J Hematol Oncol. 2021;14:156.
Yin W, Li Y, Gu Y, Luo M. Nanoengineered targeting strategy for cancer immunotherapy. Acta Pharmacol Sin. 2020;41:902–10.
Zhang Z, Wang H, Tan T, Li J, Wang Z, Li Y. Rational design of nanoparticles with deep tumor penetration for effective treatment of tumor metastasis. Adv Funct Mater. 2018;28:1801840.
Chen J, Zhu Y, Wu C, Shi J. Nanoplatform-based cascade engineering for cancer therapy. Chem Soc Rev. 2020;49:9057–94.
Castro F, Cardoso AP, Gonçalves RM, Serre K, Oliveira MJ. Interferon-gamma at the crossroads of tumor immune surveillance or evasion. Front Immunol. 2018;9:847.
Yan W, Lang T, Qi X, Li Y. Engineering immunogenic cell death with nanosized drug delivery systems improving cancer immunotherapy. Curr Opin Biotechnol. 2020;66:36–43.
Zhou L, Zhang P, Wang H, Wang D, Li Y. Smart nanosized drug delivery systems inducing immunogenic cell death for combination with cancer immunotherapy. Acc Chem Res. 2020;53:1761–72.
Li J, Wang H, Wang Y, Gong X, Xu X, Sha X, et al. Tumor-activated size-enlargeable bioinspired lipoproteins access cancer cells in tumor to elicit anti-tumor immune responses. Adv Mater. 2020;32:e2002380.
Hu M, Zhang J, Kong L, Yu Y, Hu Q, Yang T, et al. Immunogenic hybrid nanovesicles of liposomes and tumor-derived nanovesicles for cancer immunochemotherapy. ACS Nano. 2021;15:3123–38.
Löb S, Königsrainer A, Rammensee H-G, Opelz G, Terness P. Inhibitors of indoleamine-2,3-dioxygenase for cancer therapy: Can we see the wood for the trees? Nat Rev Cancer. 2009;9:445–52.
Feng B, Zhou F, Hou B, Wang D, Wang T, Fu Y, et al. Binary cooperative prodrug nanoparticles improve immunotherapy by synergistically modulating immune tumor microenvironment. Adv Mater. 2018;30:1803001.
Lang T, Zheng Z, Huang X, Liu Y, Zhai Y, Zhang P, et al. Ternary regulation of tumor microenvironment by heparanase-sensitive micelle-loaded monocytes improves chemo-immunotherapy of metastatic breast cancer. Adv Funct Mater. 2021;31:2007402.
Wang D, Wang T, Yu H, Feng B, Zhou L, Zhou F, et al. Engineering nanoparticles to locally activate T cells in the tumor microenvironment. Sci Immunol. 2019;4:eaau6584.
Wang D, Wang T, Liu J, Yu H, Jiao S, Feng B, et al. Acid-activatable versatile micelleplexes for PD-L1 blockade-enhanced cancer photodynamic immunotherapy. Nano Lett. 2016;16:5503–13.
Gao A, Chen B, Gao J, Zhou F, Saeed M, Hou B, et al. Sheddable prodrug vesicles combating adaptive immune resistance for improved photodynamic immunotherapy of cancer. Nano Lett. 2020;20:353–62.
Hou B, Zhou L, Wang H, Saeed M, Wang D, Xu Z, et al. Engineering stimuli-activatable boolean logic prodrug nanoparticles for combination cancer immunotherapy. Adv Mater. 2020;32:e1907210.
Fang L, Zhao Z, Wang J, Xiao P, Sun X, Ding Y, et al. Light-controllable charge-reversal nanoparticles with polyinosinic-polycytidylic acid for enhancing immunotherapy of triple negative breast cancer. Acta Pharmacol Sin B 2022;12:353–63.
Wang Z, Gong X, Li J, Wang H, Xu X, Li Y, et al. Oxygen-delivering polyfluorocarbon nanovehicles improve tumor oxygenation and potentiate photodynamic-mediated antitumor immunity. ACS Nano. 2021;15:5405–19.
Wang J, Meng J, Ran W, Lee RJ, Teng L, Zhang P, et al. Hepatocellular carcinoma growth retardation and pd-1 blockade therapy potentiation with synthetic high-density lipoprotein. Nano Lett. 2019;19:5266–76.
Zhou T, Liang X, Wang P, Hu Y, Qi Y, Jin Y, et al. A hepatocellular carcinoma targeting nanostrategy with hypoxia-ameliorating and photothermal abilities that, combined with immunotherapy, inhibits metastasis and recurrence. ACS Nano. 2020;14:12679–96.
Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol. 2009;10:718–26.
Jin J, Zhao Q. Engineering nanoparticles to reprogram radiotherapy and immunotherapy: Recent advances and future challenges. J Nanobiotechnology. 2020;18:75.
Wang Y, Chen J, Duan R, Gu R, Wang W, Wu J, et al. High-Z-sensitized radiotherapy synergizes with the intervention of the pentose phosphate pathway for in situ tumor vaccination. Adv Mater. 2022;34:2109726.
Feng B, Hou B, Xu Z, Saeed M, Yu H, Li Y. Self-amplified drug delivery with light-inducible nanocargoes to enhance cancer immunotherapy. Adv Mater. 2019;31:e1902960.
Zhou F, Feng B, Yu H, Wang D, Wang T, Ma Y, et al. Tumor microenvironment-activatable prodrug vesicles for nanoenabled cancer chemoimmunotherapy combining immunogenic cell death induction and CD47 blockade. Adv Mater. 2019;31:e1805888.
Morse MA, Gwin WR, Mitchell DA. Vaccine therapies for cancer: then and now. Target Oncol. 2021;16:121–52.
Saxena M, van der Burg SH, Melief CJM, Bhardwaj N. Therapeutic cancer vaccines. Nat Rev Cancer. 2021;21:360–78.
Das A, Ali N. Nanovaccine: an emerging strategy. Expert Rev Vaccines. 2021;20:1273–90.
Wang T, Wang D, Yu H, Feng B, Zhou F, Zhang H, et al. A cancer vaccine-mediated postoperative immunotherapy for recurrent and metastatic tumors. Nat Commun. 2018;9:1532.
Fang L, Zhao Z, Wang J, Zhang P, Ding Y, Jiang Y, et al. Engineering autologous tumor cell vaccine to locally mobilize antitumor immunity in tumor surgical bed. Sci Adv. 2020;6:eaba4024.
Zhou L, Hou B, Wang D, Sun F, Song R, Shao Q, et al. Engineering polymeric prodrug nanoplatform for vaccination immunotherapy of cancer. Nano Lett. 2020;20:4393–402.
Jiang Y, Krishnan N, Zhou J, Chekuri S, Wei X, Kroll AV, et al. Engineered cell-membrane-coated nanoparticles directly present tumor antigens to promote anticancer immunity. Adv Mater. 2020;32:e2001808.
Xiao P, Wang J, Zhao Z, Liu X, Sun X, Wang D, et al. Engineering nanoscale artificial antigen-presenting cells by metabolic dendritic cell labeling to potentiate cancer immunotherapy. Nano Lett. 2021;21:2094–103.
Xiao P, Wang J, Fang L, Zhao Z, Sun X, Liu X, et al. Nanovaccine-mediated cell selective delivery of neoantigens potentiating adoptive dendritic cell transfer for personalized immunization. Adv Funct Mater. 2021;31:2104068.
Liu C, Liu X, Xiang X, Pang X, Chen S, Zhang Y, et al. A nanovaccine for antigen self-presentation and immunosuppression reversal as a personalized cancer immunotherapy strategy. Nat Nanotechnol. 2022;17:531–40.
Wang J, Zheng C, Zhai Y, Cai Y, Lee RJ, Xing J, et al. High-density lipoprotein modulates tumor-associated macrophage for chemoimmunotherapy of hepatocellular carcinoma. Nano Today. 2021;37:101064.
Xu X, Gong X, Wang Y, Li J, Wang H, Wang J, et al. Reprogramming tumor associated macrophages toward M1 phenotypes with nanomedicine for anticancer immunotherapy. Adv Ther. 2020;3:1900181.
Wei Z, Zhang X, Yong T, Bie N, Zhan G, Li X, et al. Boosting anti-PD-1 therapy with metformin-loaded macrophage-derived microparticles. Nat Commun. 2021;12:440.
Rosenberg SA. IL-2: The first effective immunotherapy for human cancer. J Immunol. 2014;192:5451–8.
Propper DJ, Balkwill FR. Harnessing cytokines and chemokines for cancer therapy. Nat Rev Clin Oncol. 2022;19:237–53.
Zhai Y, Wang J, Lang T, Kong Y, Rong R, Cai Y, et al. T lymphocyte membrane-decorated epigenetic nanoinducer of interferons for cancer immunotherapy. Nat Nanotechnol. 2021;16:1271–80.
Wu T, Qiao Q, Qin X, Zhang D, Zhang Z. Immunostimulatory cytokine and doxorubicin co-loaded nanovesicles for cancer immunochemotherapy. Nanomedicine. 2019;18:66–77.
Hu Q, Shang L, Wang M, Tu K, Hu M, Yu Y, et al. Co-delivery of paclitaxel and interleukin-12 regulating tumor microenvironment for cancer immunochemotherapy. Adv Health Mater. 2020;9:e1901858.
Feng B, Niu Z, Hou B, Zhou L, Li Y, Yu H. Enhancing triple negative breast cancer immunotherapy by ICG-templated self-assembly of paclitaxel nanoparticles. Adv Funct Mater. 2020;30:1906605.
Wang H, Li J, Wang Z, Wang Y, Xu X, Gong X, et al. Tumor-permeated bioinspired theranostic nanovehicle remodels tumor immunosuppression for cancer therapy. Biomaterials. 2021;269:120609.
Acknowledgements
National Natural Science Foundation of China (81871471, 31930066, 32130058, and 32171315), Natural Science Foundation of Shanghai (19ZR1479900), Science and Technology Commission of Shanghai Municipality (19431900800), International Partnership Program of CAS (153631KYSB20190013), Natural Science Foundation of Shandong (ZR2019ZD25), Special Research Assistant Project of CAS, China Postdoctoral Science Foundation (2020M681428), and Shanghai Postdoctoral Excellence Program (2020495) are gratefully acknowledged for financial support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yan, Wl., Lang, Tq., Yuan, Wh. et al. Nanosized drug delivery systems modulate the immunosuppressive microenvironment to improve cancer immunotherapy. Acta Pharmacol Sin 43, 3045–3054 (2022). https://doi.org/10.1038/s41401-022-00976-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-022-00976-6
Keywords
This article is cited by
-
Gemcitabine-loaded synthetic high-density lipoprotein preferentially eradicates hepatic monocyte-derived macrophages in mouse liver with colorectal cancer metastases
Acta Pharmacologica Sinica (2023)
-
APS celebrates the 90th anniversary of SIMM
Acta Pharmacologica Sinica (2022)