Abstract
Hepatic steatosis plays a detrimental role in the onset and progression of alcohol-associated liver disease (ALD). Mesencephalic astrocyte-derived neurotrophic factor (MANF) is an evolutionarily conserved protein related to the unfolded protein response. Recent studies have demonstrated that MANF plays an important role in liver diseases. In this study, we investigated the role of MANF in ethanol-induced steatosis and the underlying mechanisms. We showed that the hepatic MANF expression was markedly upregulated in mouse model of ALD by chronic-plus-single-binge ethanol feeding. Moreover, after chronic-plus-binge ethanol feeding, hepatocyte-specific MANF knockout (HKO) mice displayed more severe hepatic steatosis and liver injury than wild-type (WT) control mice. Immunoprecipitation-coupled MS proteomic analysis revealed that arginosuccinate synthase 1 (ASS1), a rate-limiting enzyme in the urea cycle, resided in the same immunoprecipitated complex with MANF. Hepatocyte-specific MANF knockout led to decreased ASS1 activity, whereas overexpression of MANF contributed to enhanced ASS1 activity in vitro. In addition, HKO mice displayed unique urea cycle metabolite patterns in the liver with elevated ammonia accumulation after ethanol feeding. ASS1 is known to activate AMPK by generating an intracellular pool of AMP from the urea cycle. We also found that MANF supplementation significantly ameliorated ethanol-induced steatosis in vivo and in vitro by activating the AMPK signaling pathway, which was partly ASS1 dependent. This study demonstrates a new mechanism in which MANF acts as a key molecule in maintaining hepatic lipid homeostasis by enhancing ASS1 activity and uncovers an interesting link between lipid metabolism and the hepatic urea cycle under excessive alcohol exposure.
Similar content being viewed by others
Introduction
Alcohol-associated liver diseases (ALDs) exhibit a wide spectrum of disease severities from fatty liver to cirrhosis. Fatty liver or ectopic lipid deposition in hepatocytes is not only the most prevalent and earliest reaction to alcohol insults but is also a critical step for liver cancer development [1]. Hepatocytes are a pivotal hub of the metabolism of glucose, lipids, and proteins. The liver orchestrates the interaction or crossover regulation among nutrient metabolism [2]. The urea cycle (UC) was the first identified metabolic cycle and is considered an essential step to eliminate ammonia, the “waste” product generated during protein catabolism [3]. The hepatic urea cycle consists of five enzymatic reactions, and arginosuccinate synthase 1 (ASS1), with the lowest Vmax, is considered the rate-limiting step in the cycle [4]. The hepatic urea cycle is mediated by two mitochondrial enzymes, carbamoyl phosphate synthase 1 (CPS1) and ornithine transcarbamylase (OTC), and three cytoplasmic enzymes, ASS1, arginosuccinate lyase (ASL) and arginase (ARG). ASS1 mediates citrulline and aspartate condensation and produces arginosuccinate acid, the precursor of arginine. Its enzymatic activity is affected by nutrients [5] and protein posttranslational modifications [6]. Patients with UC defects display subtype-specific changes in the involved metabolites [7]. ARG-deficient patients exhibit elevated arginine and argininosuccinate levels [8]. ASS-deficient patients are characterized by mild citrullinemia [9]. Associations between a disordered urea cycle and dyslipidemia have been observed in various liver diseases. Patients with urea cycle disorders (UCDs), such as citrulline type I disease, develop steatosis-like diseases [10]. In mouse models of nonalcoholic fatty liver disease (NAFLD), liver steatosis led to UC dysfunction and worsened fibrosis progression due to the intrahepatic accumulation of toxic ammonia [11]. In patients with alcoholic hepatitis (AH), the hepatic nitrogen clearance rate is significantly decreased, and urea synthetic capacity decreases with increased severity of AH, but the specific underlying mechanism or ethanol’s influence on ASS1 enzymatic activity remains unknown [12].
Mesencephalic astrocyte-derived neurotrophic factor (MANF) was named based on its originally identified role as a neuroprotective factor [13]. Subsequent studies demonstrated that MANF participates in protein folding in the endoplasmic reticulum (ER) lumen [14] and acts as an anti-inflammatory factor against the NF-κB signaling pathway [15]. The cytoprotective effects of MANF have been demonstrated in studies using MANF knockout animal models. MANF knockout in the pancreas and β-cells leads to postnatal depletion of β-cells and diabetes. Global MANF deficiency in mice results in severe diabetes-independent growth retardation [16]. Studies using conditional deletion of MANF from the neural lineage of cells in the CNS demonstrated that MANF is not needed for the proper function of midbrain dopamine neurons in the aging brain despite the upregulation of unfolded protein response (UPR)-associated genes [17]. MANF also plays important roles in the regulation of inflammation and promotes tissue repair and regeneration by directly acting on immune cells [18]. MANF can act directly on macrophages to modulate their inflammatory phenotype by reducing proinflammatory signaling and promoting reparative activation in response to retinal damage in flies and mice [19]. Mono-macrophage-specific MANF knockout mice exhibited increased splenic proinflammatory (M1-like) macrophages but no significant change in splenic morphology or size compared to wild-type mice [20]. MANF was also reported to play an important role in lipid metabolism [21]. HepG2 cells were more likely to develop steatosis after MANF knockdown in free fatty acid-supplemented culture conditions [22]. MANF overexpression in HepG2 cells resulted in repression of G0S2, an inhibitory regulator of lipolysis [23]. However, there remain many unknowns regarding the role and underlying mechanisms of MANF in lipid metabolism disorders in liver disease. Our previous studies found that hepatic expression of MANF in female C57BL/6J mice was increased after induction of the Gao–Binge ethanol model of ALD. Hepatocyte-specific knockout of MANF deteriorates alcohol-induced liver injury accompanied by overactive ER stress [24]. However, the role of MANF in substance metabolism in the liver as well as the associated mechanisms remains to be elucidated in ALD. The present study aimed to explore MANF’s role in ethanol-induced steatosis and the underlying mechanisms.
Materials and methods
Animals and the Gao–Binge ethanol model
Male C57BL/6J mice (6–8 weeks, 20–25 g) were obtained from Jiangsu Jicui Yaokang Biotechnology Co., Ltd. MANFflox/flox mice on the C57BL/6J background bearing loxP sites flanking exon 3 of the MANF gene were cross-bred with Alb-cre mice to generate hepatocyte-specific MANF knockout (HKO) mice. The efficiency of MANF knockout in HKO mouse livers was validated by Western blot [25]. Mice were maintained under specific pathogen-free conditions on a standard 12 h/12 h light cycle. Distilled water and rodent chow (Shoobree, Nanjing, China) were provided ad libitum. All procedures (LLSC20210761) were approved by the Institutional Animal Care and Use Committee (IACUC) of Anhui Medical University. The Gao–Binge ethanol model was established according to the NIAAA protocol [26]. In brief, mice were fed a control Lieber–DeCarli diet (Trophic Animal Feed High-Tech Co. Ltd.) for 5 days to acclimatize them to a liquid diet. During the following 10 days, mice were fed a Lieber–DeCarli ethanol diet. Mice in the control group were pair-fed an isocaloric control diet. On the morning of day 11, a single dose of ethanol (5 g/kg body weight) or isocaloric dextrin maltose was administered via oral gavage to mice in ethanol- or control-fed groups, respectively. Mice were euthanized 9 h later for tissue collection.
Cell culture and treatment
HEK293T cells (ATCC CRL-3216) and HepG2 cells (ATCC HB-8065) were cultured in DMEM supplemented with 10% fetal bovine serum (Gibco), penicillin−streptomycin (Beyotime), and 2 mM L-glutamine (Beyotime). Cells were grown on tissue culture dishes or in multiwell plates (Costar) at 37 °C and 5% CO2. Primary mouse hepatocytes were isolated using a two-step collagenase approach [27] and were subjected to ASS1 activity assay, immunoblotting or qRT−PCR.
Protein expression of recombinant human MANF (rhMANF) from E. coli BL21 and purification were performed as previously described [28]. To determine the effect of rhMANF on ethanol-induced lipid droplet (LD) accumulation in vitro, HepG2 cells were starved in serum-free medium for 16 h before the experiment. HepG2 cells were then treated with 100 mM ethanol with or without rhMANF supplementation for the indicated period. After treatment, the cells were washed and lysed in cell lysis buffer (Beyotime) supplemented with protease and phosphatase inhibitor cocktail (Beyotime) on ice. Total protein of the cell lysates was quantified using a BCA kit (Thermo Scientific), and equal amounts of cellular proteins from each treatment group were subjected to Western blot analysis. The primary antibodies and dilution ranges used for immunoblotting are summarized in Supplementary Table 1. Band densities were quantified using ImageJ software [29]. The intracellular accumulation of lipid droplets was evaluated using Oil Red O staining (Beyotime). Three representative images from each treatment group were used for quantification of the percentage of Oil Red O staining area using ImageJ. Data are expressed as the average values ± standard deviation (SD). For transient knockdown of ASS1, a set of three siRNAs targeting the human ASS1 gene and the negative control siRNA were purchased (Suzhou GenePharma). siRNAs were tested on HepG2 cells, and the most efficient siRNA 3 [CCC GCA AAC AAG UGG AAA UTT (sense)] (Supplementary Fig. 1), was used to specifically downregulate ASS1 expression in subsequent experiments.
Immunoprecipitation-coupled mass spectrometry analysis
Immunoprecipitation was performed on HepG2 cell lysates using anti-MANF antibody (ab67271, Abcam) or isotype control antibody (CST3900, Cell Signaling Technology). Proteins were subjected to SDS−PAGE separation, and the excised gel was sent to Shanghai Applied Protein Technology Company for downstream analysis. In brief, LC−MS (Q Exactive™ Quadrupole-Orbitrap™ Mass Spectrometer and Easy-nLC 1000 chromatograph, Thermo Fisher) was performed on the peptide mixture obtained from trypsin digestion of protein samples. The peptide sequence was identified by bioinformatics analysis using MASCOT software. Unique peptides of ASS1 identified by mass spectrometry are listed in Fig. 3a. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the iProX partner repository [30] with the dataset identifier PXD031750.
Verification of the MANF and ASS1 interaction via coimmunoprecipitation analysis
HEK293T cells were transfected with pClneo-MANF-FLAG using Lipofectamine™ 2000 (Thermo Fisher Scientific). Whole-cell lysates were prepared 72 h posttransient transfection in lysis buffer containing 50 mM HEPES (pH 7.4), 150 mM NaCl, 0.1% Triton X-100, 2 mM EGTA and protease inhibitor cocktail (Beyotime). Proteins were extracted, incubated with anti-MANF antibody (ab67271, Abcam) or anti-Flag antibody (F1804, Sigma Aldrich) and immunoprecipitated using protein-A/G Plus agarose beads (sc-2003, Santa Cruz Biotechnology). Eluted samples were subjected to SDS−PAGE separation. Blots were probed using anti-ASS1 (16210-1-AP, Proteintech) or anti-MANF antibody (ab126321, Abcam).
ASS1 activity assay
The ASS1 activity assay was conducted following a previously described protocol [31] with modification. In brief, frozen cells were resuspended in lysis buffer (50 mM Tris-HCl, pH 8.0, 10% glycerol, protease inhibitor cocktail) and then incubated on ice for 5 min. After centrifugation for 20 min at 12,000 ×g at 4 °C, 10 μg protein from the supernatants was added to the reaction buffer (20 mM Tris-HCl, pH 7.8, 2 mM adenosine 5’-triphosphate, 2 mM citrulline, 2 mM aspartate, 6 mM MgCl2, 20 mM KCl, and 0.1 U pyrophosphatase) to a final volume of 0.1 mL. Samples were incubated for 60 min at 37 °C. The reactions were stopped by the addition of an equal volume of molybdate buffer (10 mM ascorbic acid, 2.5 mM ammonium molybdate, and 2% sulfuric acid). The accumulation of pyrophosphate, a byproduct of arginosuccinate synthesis, was measured spectrophotometrically at 660 nm, and the quantitation was performed based on an inorganic phosphate standard curve.
Metabolite measurement
Collected mouse liver tissues were snap frozen in liquid nitrogen and homogenized using a cryogenic grinder (JXFSTPRP-CL, Shanghai Jing Xin Industrial Development CO., LTD) in extraction buffer containing 20% methanol, 0.1% formic acid, 3 mM NaF and 100 μM EDTA. Liver homogenates were centrifuged at 12,000 ×g for 20 min at 4 °C, and the protein concentration was measured using a BCA assay. Supernatant samples were mixed with two volumes of 70% acetonitrile and filtered through a 0.22 μm syringe filter before use. The separation was performed on a Waters ACQUITY UPLC NH2 column (1.8 μm particle size, 2.1 mm × 100 mm) at a flow rate of 0.4 mL/min using Dionex Ultimate 3000 ULTRA High Performance Liquid Chromatography (Thermo Fisher Scientific). Isocratic mobile solvents consisting of 65% (vol/vol) acetonitrile and 35% (vol/vol) water containing 0.1% formic acid were used to achieve ideal retention time from chromatographic separation using known standards. The samples were ionized by a heated electrospray ion source (HESI) and collected in positive ion scanning mode using targeted selected ion monitoring (SIM) mode: 176.2 for citrulline, 134.1 for aspartic acid, 133.1 for ornithine, and 175.2 for arginine. Xcalibur 4.1 software (Thermo Fisher Scientific) was used for data acquisition and analysis.
Blood analysis
Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), total cholesterol (TC), and total triglyceride (TG) levels and blood urea nitrogen (BUN) were quantified on a Hitachi automatic analyzer 3100 according to the manufacturer’s manuals.
Liver tissue analysis
Hepatic expression of MANF was evaluated using IHC, Western blot or qRT−PCR analysis. Briefly, formalin-fixed, paraffin-embedded liver sections were dewaxed and rehydrated. Antigen retrieval was conducted using proteinase K treatment. Furthermore, endogenous peroxidase activity was quenched in 0.3% H2O2. Sections were incubated with primary antibodies overnight at 4 °C. After incubation with the secondary antibodies, immunostaining was developed using a DAB substrate solution. Moreover, sections were counterstained with hematoxylin. Details on the primary antibodies are listed in Supplementary Table 1. Total RNA from frozen liver tissues was extracted using TRIzol reagent (Invitrogen). RNA was further reverse transcribed into cDNA. Target gene transcripts were quantified using real-time quantitative PCR with SYBR green on the ABI 7500 real-time PCR detection system (Applied Biosystems by Thermo Fisher Scientific). The primers used to amplify mRNA for qRT−PCR are listed in Supplementary Table 2. Ammonia accumulation in mouse liver sections was detected using Nessler’s staining reagent (Supelco1090280, Sigma). Fifty to 100 mg of frozen liver tissue was homogenized at 4 °C in ethanol (w:v = 1:9) using an automatic sample freezer grinder (Shanghai Jingxin JXFSTPRP-CL). After centrifugation at 12,000 rpm for 15 min, the supernatant was used to measure TC and TG levels on a biochemical analyzer (Hitachi 3100).
Statistical analysis
All data are expressed as the mean ± SD. Student’s t tests were performed on data for comparisons between two groups. For multigroup comparisons, statistical analysis was calculated by one-way ANOVA in GraphPad Prism 9.0 software (GraphPad Software Inc., CA, USA) with the least-significant difference test as a post hoc test for comparing the differences between groups on quantified data. P values less than 0.05 were considered statistically significant.
Results
Hepatic expression of MANF is upregulated in the Gao–Binge ethanol model
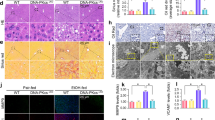
The role of MANF in liver diseases has received growing research interest. MANF is highly expressed in metabolically active tissues, including the pancreas, liver, and hypothalamus [32]. Hepatic MANF is primarily expressed in hepatocytes, macrophages, and neutrophils but not in hepatic stellate cells [33]. Hepatic MANF expression was also increased under inflammatory conditions, which was supported by the observed upregulation of MANF expression in liver tissues from patients with HBV infection [34]. In this study, our initial goal was to characterize MANF expression changes in alcohol-associated liver disease. The Gao–Binge ethanol model was applied following the well-established NIAAA protocol to develop a mouse model of ALD. Only male mice were used to avoid potential confounding effects on lipid metabolism due to hormone fluctuations [35]. The mRNA and protein levels of MANF in the liver tissues of ethanol-fed mice were significantly increased compared to those in the control diet group (Fig. 1a, b). Upregulation of MANF protein was primarily observed in hepatic parenchymal cells (Fig. 1c).
a Western blot analysis of MANF protein levels in mouse livers. MANF bands were normalized to GAPDH band density. The quantitative blot data are expressed in a scatter plot. Data are expressed as the average values, and error bars indicate the standard deviation (SD). b Relative mRNA level of MANF in WT mouse livers. Data are presented as relative expression in the EtOH-fed group compared to the control-fed group using the 2−∆∆Ct method. c Immunostaining for MANF in paraffin sections of mouse livers. Scale bar = 20 μm. Data marked with asterisks indicate statistically significant differences compared to the pair-fed group using Student’s t test. **P < 0.01; ***P < 0.001.
MANF deficiency in hepatocytes promotes liver steatosis in the Gao–Binge ethanol model
Hepatic steatosis plays a detrimental role in the onset and progression of alcohol-associated liver disease, which prompted us to evaluate the potential influence of hepatocyte-derived MANF on ethanol-induced steatosis in an ALD model. We used HKO mice to establish the Gao–Binge ethanol model. Ethanol-induced LD deposition in the liver of the WT group caused mild steatosis. In contrast, a large number of LDs and ballooning of hepatocytes were observed in the livers of HKO mice (Fig. 2c, h, i). In addition, serum AST levels after ethanol feeding in HKO mice were significantly higher than those in ethanol-fed WT mice (Fig. 2a). Ethanol feeding also led to increased levels of total TG in serum and in liver lysates in both HKO mice and WT mice, whereas the TG increase was more pronounced in HKO mice (Fig. 2d, e). Together, these results indicate that hepatocyte MANF deficiency made the liver more susceptible to ethanol-induced liver injury and steatosis. Notably, serum ALT levels were not significantly different between ethanol-fed WT and ethanol-fed HKO mice in this study (Fig. 2b). However, we observed significantly higher ALT levels in female HKO mice after ethanol feeding than in WT mice [24]. This discrepancy may partly be due to sex differences, as female rodents are more susceptible to ethanol-induced liver injury [36]. It was also noted that serum levels of TC were similar between WT and HKO mice. We did not observe serum TC changes after ethanol feeding in mice. However, baseline levels of hepatic TC were mildly elevated in HKO mice compared to WT mice, but the specific reasons are not clear and remain to be elucidated (Fig. 2f, g).
a, b Serum levels of the hepatic function indicators AST and ALT, respectively. c Representative histological images stained with hematoxylin and eosin (H&E). Scale bar = 20 μm. d–g Detection of TG and TC concentrations in serum and liver tissue. h, i Representative Oil Red O staining images of liver sections from mice in the control-fed or ethanol-fed group. Scale bar = 50 μm. Quantification of the percentage of Oil Red O staining area was performed using ImageJ. Data are expressed as the average values ± SD. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001 between the indicated groups.
MANF resides in the same immunoprecipitated complex as ASS1 and positively regulates ASS1 activity
Immunoprecipitation-mass spectrometry (IP-MS) is a dedicated tool to identify protein−protein interactions [37]. IP-MS-based proteomics was used to identify the potential interacting partners of MANF. Mass spectrometry results suggested that ASS1, a rate-limiting enzyme in the urea cycle, exhibited potential protein−protein interactions with MANF (Fig. 3a). To validate the screening result, we conducted a coIP experiment using HEK293T cells overexpressing the MANF-Flag protein. The immunoblot results indicated that MANF and ASS1 resided in the same immunoprecipitated complex (Fig. 3b). ASS1 is a rate-limiting enzyme of the urea cycle that catalyzes the conversion of citrulline and aspartate to arginosuccinate. To elucidate the potential impacts of MANF on ASS1, the expression and enzyme activity of ASS1 were evaluated. In response to MANF knockout, both the mRNA and protein levels of ASS1 in the liver tissues of HKO mice were slightly increased (Fig. 3d, e). Next, we isolated primary hepatocytes from HKO and WT mice to compare ASS1 enzyme activity. The results showed that ASS1 activity was reduced in MANF-deficient hepatocytes (Fig. 3c). In MANF-overexpressing HEK293T cells, ASS1 displayed enhanced activity even though the ASS1 protein remained at a similar expression level (Fig. 3f, g). Taken together, these results suggest that MANF positively regulates ASS1 enzyme activity rather than directly mediating ASS1 expression. The levels of urea cycle metabolites in liver homogenates were then quantified using an LC/MS approach. CIT, ORN and ARG, but not ASP, were elevated in HKO livers, suggesting a unique urea cycle metabolite pattern in HKO livers (Fig. 3h). The outcome of the altered metabolite pattern on liver physiology in HKO mice or whether the metabolite level changes are related to pathological interference in ALD remains to be elucidated [3]. Notably, citrulline, a direct substrate of ASS1, was increased in HKO mouse livers. This observation is consistent with the elevated citrulline levels observed in clinical ASS deficiency [9] and ASS1 knockdown animal models [38].
a Tryptic peptide of ASS1 identified by mass spectrometry in immunoprecipitated HepG2 cell lysates using an anti-MANF antibody. b HEK293T cells overexpressing MANF-Flag protein were used in the coimmunoprecipitation assay. c ASS1 enzymatic activity was decreased in isolated primary hepatocytes from HKO mice compared to those from WT mice. d, e Both ASS1 mRNA and protein levels were slightly increased in isolated primary hepatocytes from HKO mice compared to WT mice. f ASS1 enzymatic activity was increased in HEK293T cells overexpressing MANF compared to the mock-transfected control. g ASS1 expression was not altered in MANF-overexpressing HEK293T cells. h Urea cycle metabolites in HKO mouse livers and WT mouse livers were quantified using LC/MS. Data marked with asterisks indicate statistically significant differences between the indicated groups. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001 between the indicated groups.
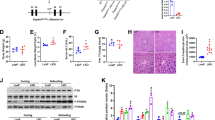
MANF mitigates liver steatosis by counteracting alcohol-induced AMPK inactivation
ASS1 mediates the conjugation of citrulline and aspartate acid into arginosuccinate with the consumption of ATP and converts ATP to AMP [4]. Under protein intake or amino acid influx, ASS1 links protein catabolism to AMPK activation by generating an intracellular AMP pool in hepatocytes [38]. Alcohol-induced liver damage is closely related to the AMPK-mediated lipid metabolism pathway. Ethanol reduces AMPK activity by increasing the dephosphorylation of AMPK [39]. We next investigated whether the MANF and ASS1 interaction impacted the AMPK signaling pathway. There was no significant difference in hepatic expression levels of AMPK between the WT and HKO groups (Fig. 4a, b). Furthermore, AMPK expression was not altered in the Gao–Binge model. However, the ratio of active phosphorylated AMPK (phospho-AMPK Thr172) was decreased after ethanol intake, supporting that ethanol exerts negative effects on AMPK activation. After hepatocyte MANF knockout, the phosphorylation ratio of AMPK in liver tissues was decreased. Ethanol led to a more pronounced decrease in AMPK phosphorylation in HKO mice than in WT mice. Transcription levels of AMPK downstream genes associated with lipid metabolism, such as screbp1c and fasn, were also significantly increased after MANF knockout, whereas ACC1 (acaca) mRNA levels did not change (Fig. 4c). Moreover, ethanol intake led to increased levels of the screbp1c, fasn and ACC1 mRNAs in both WT and HKO mouse livers. Taken together, these results indicate that alcohol interrupts the AMPK signaling pathway partly, if not all, through its association with MANF. Chronic plus binge ethanol intake also induced increased levels of serum urea in WT mice (Fig. 4d). This might be due to binge ethanol intake, which inhibits protein synthesis in peripheral muscle tissues and promotes nitrogen clearance [40]. However, ethanol-induced ureagenesis was not observed in HKO mice. These results suggest that increased ureagenesis in the liver after ethanol intake is associated with the expression of MANF; that is, loss of MANF weakens the adaptive response of the liver by increasing urea synthesis after alcohol intake. In addition, hepatic accumulation of ammonia due to a compromised urea cycle causes liver injury [11, 41]. Nessler’s staining results revealed that ethanol intake led to increased accumulation of ammonia in liver sections from HKO mice compared to those from WT mice (Fig. 4e). Therefore, we speculated that MANF deficiency compromises urea synthesis in hepatocytes and leads to increased “toxic” ammonia levels in the liver upon ethanol-induced nitrogen clearance. This may also be one of the factors in addition to steatosis that contribute to the more pronounced ethanol-induced liver damage observed in HKO mice.
a, b Western blot analysis of AMPK and phosphorylated AMPK in mouse livers. The quantitative blot data are expressed in a scatter plot. c The mRNA levels of AMPK-downstream genes in lipid metabolism. d BUN levels were quantified on a Hitachi automatic analyzer 3100 according to the manufacturer’s manuals. e The accumulation of ammonia in liver sections was detected using Nessler’s reagent. Scale bar = 20 μm. Data marked with asterisks indicate statistically significant differences between the indicated groups. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ns not significant.
Next, we supplemented HKO mice with recombinant human MANF protein (2 mg/kg body weight) by intraperitoneal injection starting from diet acclimation until the last day of ethanol feeding. The phosphorylation ratio of AMPK increased after MANF supplementation compared to that in the vehicle treatment group (Fig. 5a). Hepatic levels of TC and TG were also reduced (Fig. 5c). Oil Red O staining results indicated that MANF supplementation alleviated ethanol-induced LD accumulation in HKO mice (Fig. 5b, d). The effect of MANF on ethanol-induced AMPK deactivation and LD accumulation was then evaluated in vitro. HepG2 cells were treated with 100 mM ethanol over 48 h. Recombinant human MANF protein at doses ranging from 3, 10–30 µg/mL was supplemented in the medium along with ethanol treatment. Ethanol treatment led to decreased AMPK phosphorylation ratios compared to the vehicle control. MANF supplementation reduced ethanol-induced AMPK dephosphorylation in a dose-dependent manner up to 10 µg/mL (Fig. 5e). In addition, MANF reduced ethanol-induced LD accumulation in HepG2 cells (Fig. 5f, g). Steatosis in early ALD is a reversible process. Therefore, we evaluated whether MANF supplementation was able to aid in the removal of LDs after ethanol insults. MANF supplementation facilitated the removal of LDs and AMPK recovery in HepG2 cells after a 48-h ethanol challenge (Supplementary Fig. 3a, b). To evaluate the role of ASS1 in the regulation of AMPK by MANF, HepG2 cells were transfected with ASS1 siRNA. ASS1 knockdown in HepG2 cells was confirmed by Western blot analysis (Supplementary Fig. 2). ASS1 knockdown significantly weakened the MANF mitigation of ethanol-induced AMPK deactivation and LD accumulation (Fig. 6a–d). These results indicate that MANF’s effect on AMPK activation is partly, if not entirely, mediated by its interaction with ASS1.
a Western blot analysis of AMPK and phosphorylated AMPK in HKO mouse livers after Gao–Binge ethanol feeding. HKO mice were supplemented with rhMANF (2 mg/kg body weight) or vehicle control by intraperitoneal injection starting from diet acclimation until the last day of ethanol feeding. b–d Supplementation with rhMANF protein led to reduced hepatic accumulation of TG and TC and attenuated lipid droplet accumulation in HKO mice after chronic plus binge ethanol intake (Oil Red O staining, scale bar = 50 μm). e Western blot analysis of AMPK and phosphorylated AMPK in HepG2 cells treated with 100 mM ethanol over 48 h. rhMANF protein at a dose range from 3,10 to 30 µg/mL was supplemented in the medium in combination with ethanol treatment. f, g Lipid droplet accumulation in HepG2 cells was detected using Oil Red O staining (scale bar = 20 μm). Quantification of the percentage of Oil Red O staining area using ImageJ. Data are expressed as the average values ± SD. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001; ns not significant.
a, b Western blot analysis of AMPK and phosphorylated AMPK in ASS1 knockdown HepG2 cells. c, d Lipid droplet accumulation was detected using Oil Red O staining (scale bar = 20 μm). Quantification of the percentage of Oil Red O staining area was performed using ImageJ. Data are expressed as the average values ± SD. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001; ns not significant.
Discussion
Steatosis is the earliest liver response to long-term excess alcohol intake [42]. The degree of steatosis is an early predictor of the overall status of ALD [43]. Ethanol perturbs almost all aspects of hepatic lipid metabolism. The primary mechanisms whereby this occurs include an altered intracellular redox environment during alcohol metabolism and changes in the transcription or enzyme activities of key proteins in lipid metabolism, such as decreased activation of AMPK [39]. Ectopic fat deposition in hepatocytes contributes to other metabolic disorders of glucose, protein and inflammation, making the liver more susceptible to alcohol-induced damage [44]. Therefore, identifying key factors in the underlying mechanisms of ethanol-induced steatosis sheds light on new therapeutic strategies for ALD, especially through early intervention.
Recently, the role of MANF in maintaining liver metabolic homeostasis under conditions of obesity, aging, and inflammation has attracted growing research interest. MANF has been demonstrated to reduce cell apoptosis by responding to injury through UPR [45]. In addition, MANF plays an anti-inflammatory role by negatively regulating the NF-κB pathway [25]. Therefore, MANF may play a protective role in hepatocytes in alcohol-induced liver injury through a variety of mechanisms, and a specific and better understanding of these mechanisms remains to be elucidated.
In this study, hepatic MANF protein and mRNA levels were upregulated in the Gao–Binge ethanol model. These results are consistent with clinical observations that the transcription of MANF is upregulated in liver tissues from patients with early alcoholic steatohepatitis [46]. MANF is an ER stress-related protein, and two ER stress-responsive elements (ERSEs) have been found in the promoter region of MANF [47]. Spliced X-box binding protein-1 (XBP1s), a signature upregulated protein in the UPR triggered by ER stress, primarily binds to ERSE I to promote MANF transcription. MANF can also interact with XBP1s to enhance its own expression [34]. ER stress-induced upregulation of MANF expression is likely a feedback regulatory mechanism to maintain ER homeostasis [48]. Alcohol induces ER stress in hepatocytes, and the consequent UPR contributes to upregulating MANF expression [24, 49].
To explore the role of MANF in ethanol-induced steatosis, hepatocyte-specific MANF knockout mice were used to implement the Gao–Binge ethanol model. HKO mice displayed more severe steatosis after chronic-plus-binge ethanol feeding, while MANF supplementation to HKO mice alleviated liver steatosis in the Gao–Binge model. The antisteatosis effects of MANF are consistent with studies using liver-specific overexpressing MANF mice [50] and MANF-overexpressing HepG2 cells [22]. To investigate the underlying mechanism, IP-MS-based proteomics analysis was performed to explore the interacting partners with MANF. IP-MS results indicated that ASS1 existed in the same immunoprecipitated complex as MANF. In addition, ASS1 activity in primary hepatocytes from HKO mice was significantly reduced compared to that in primary hepatocytes from WT control mice. Citrulline, the direct substrate of ASS1, was elevated in HKO mouse livers along with a distinct UC metabolite pattern.
Overexpression of MANF led to enhanced ASS1 activity in vitro. ASS1 activates AMPK by generating an intracellular pool of AMP in hepatocytes [38]. Therefore, we hypothesized that the MANF-ASS1 interaction could ameliorate ethanol-induced steatosis in the liver by activating the AMPK signaling pathway. In this study, hepatocyte MANF deficiency exacerbated ethanol’s inhibitory effects on the AMPK-mediated lipid metabolism pathway, while MANF supplementation in HKO mice counteracted AMPK deactivation and alleviated liver steatosis in the Gao–Binge model. MANF supplementation also preserved phosphorylated AMPK levels in HepG2 cells and exerted a dose-dependent inhibitory response against ethanol-induced LD accumulation. The antisteatosis effects of MANF were significantly weakened after silencing ASS1 in HepG2 cells. These results indicate that MANF mediates the AMPK pathway partly through ASS1. Of note, phosphorylated AMPK in the WT mouse livers was decreased by ethanol administration even though the expression of hepatic MANF was upregulated. This result indicated that the increased expression of endogenous MANF was not able to completely reverse the deactivation of AMPK signaling by ethanol. Ethanol has been demonstrated to inhibit AMPK phosphorylation, and the mechanisms involve activation of protein phosphatase 2 A (PP2A) [51] and/or inhibition of the upstream AMPK kinase LKB1 [52]. Therefore, ASS1-mediated AMPK activation resulting from the limited increase in endogenous MANF was not sufficient to counteract all the inhibitory mechanisms on AMPK by ethanol. As we were finalizing our manuscript, the J. He group reported that transgenic mice overexpressing MANF in the liver exhibited reduced ethanol-induced lipid accumulation by promoting autophagy [50]. AMPK can trigger autophagy by activating ULK1 and inhibiting the suppressive effect of mTORC1 [53]. Therefore, our findings that MANF positively regulates the AMPK pathway might contribute to MANF’s regulation of autophagy to alleviate lipid droplet accumulation after ethanol feeding. Our previous studies found that ethanol induced more severe oxidative stress in HKO mice and that aggravated ER stress may also contribute to ethanol-induced liver steatosis [24]. Therefore, the relative partition of an ER stress-dependent protective mechanism of MANF or an ER stress-independent mechanism of MANF in regulating hepatic lipid metabolism remains to be elucidated. In addition, the elevated accumulation of toxic ammonia in HKO mouse livers indicates that another risk factor led to more serious liver injury after MANF knockout.
ASS1 is a cytosolic enzyme, and the partition of its association with the outer mitochondrial membrane is influenced by developmental stage and is cell type-dependent [4]. ASS1 activity is primarily mitochondrial in fetal and newborn rat livers and becomes cytoplasmic in adult rat livers [54]. Intracellular MANF is primarily localized in the ER lumen, and secretion of MANF is minimal under normal conditions [28]. However, MANF expression and its subcellular translocation are greatly affected by pathological conditions, including ER stress or inflammation [48]. ER stress significantly promotes the secretion of MANF, and current hypotheses involve the KDEL competition model, which accounts for reduced MANF retrieval from the cis-Golgi [55] and a calcium-dependent interaction with the ER-resident protein GRP78 [56]. In addition, the reverse transport of proteins from the ER to the cytoplasm is an important adaptive response for eliminating misfolded proteins under ER stress [57]. Inflammation also induces the nuclear transport of MANF and triggers consequent interaction with the NF-κB p65 subunit, which negatively regulates the NF-κB signaling pathway [15]. Moreover, released MANF can enter cells by binding to receptors such as neuroplastin (NPTN) or through endocytosis by binding to lipid sulfatide [58, 59]. Immunoblotting results from the coimmunoprecipitated sample supported the interaction between ASS1 and MANF. However, whether this interaction is direct or indirect, as MANF interacts with ASS1 in a larger immunocomplex with other partners, remains to be investigated. The mechanism through which MANF augments ASS1 enzyme activity requires in-depth exploration. The specific subcellular location where this interaction occurs, especially in hepatocytes under ethanol stimulus, also remains unclear. Considering the pathological condition-related dynamic variations in the subcellular distribution of MANF and ASS1, additional study requires a more sophisticated in vitro model together with real-time imaging studies [60].
In summary, we found that hepatic expression of MANF is upregulated in the Gao–Binge mouse model. Hepatocyte-specific MANF knockout mice displayed more severe steatosis and liver injury after chronic plus binge ethanol feeding. ASS1, a rate-limiting enzyme in the urea cycle, was identified in the immunoprecipitated complex with MANF using IP-MS. Hepatocyte-derived MANF, acting as a positive regulator of ASS1 activity, plays a protective role against ethanol-induced liver steatosis in the Gao–Binge ethanol model via the AMPK pathway (Fig. 7). This study not only demonstrated that MANF participates in liver lipid metabolism by interacting with ASS1 but also interpreted the role of increased MANF expression under alcohol stimulation in the “self-protection” mechanism of hepatocytes. Moreover, it may help to support the future application of MANF being translated into targeted therapies to treat ALD.
Ethanol perturbs almost all aspects of hepatic lipid metabolism. After long-term excessive drinking, the activity of AMPK in liver cells is inhibited, and the expression and activity of SREBP-1c and FASN increase, which induces fat deposition in liver cells. On the other hand, alcohol induces ER stress in hepatocytes, and the consequent UPR leads to upregulated MANF expression. Hepatocyte MANF deletion deteriorates ethanol-induced steatosis and suppresses the AMPK pathway in response to ethanol. MANF interacts with ASS1 and enhances ASS1 enzyme activity. ASS1 activates AMPK by generating an intracellular pool of AMP from the urea cycle. MANF mitigates ethanol-induced liver steatosis by activating the AMPK signaling pathway.
References
Yang L, Yang C, Thomes PG, Kharbanda KK, Casey CA, McNiven MA, et al. Lipophagy and alcohol-induced fatty liver. Front Pharmacol. 2019;10:495.
Bechmann LP, Hannivoort RA, Gerken G, Hotamisligil GS, Trauner M, Canbay A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol. 2012;56:952–64.
Keshet R, Szlosarek P, Carracedo A, Erez A. Rewiring urea cycle metabolism in cancer to support anabolism. Nat Rev Cancer. 2018;18:634–45.
Husson A, Brasse-Lagnel C, Fairand A, Renouf S, Lavoinne A. Argininosuccinate synthetase from the urea cycle to the citrulline-NO cycle. Eur J Biochem. 2003;270:1887–99.
Morris SM Jr. Regulation of enzymes of the urea cycle and arginine metabolism. Annu Rev Nutr. 2002;22:87–105.
Lin R, Mo Y, Zha H, Qu Z, Xie P, Zhu ZJ, et al. CLOCK acetylates ASS1 to drive circadian rhythm of ureagenesis. Mol Cell. 2017;68:198–209.e6.
Blau N, Duran M, Blaskovics ME, Gibson KM. Physician’s guide to the laboratory diagnosis of metabolic diseases. Springer Science & Business Media; 2003.
Peng MZ, Cai YN, Shao YX, Zhao L, Jiang MY, Lin YT, et al. Simultaneous quantification of 48 plasma amino acids by liquid chromatography-tandem mass spectrometry to investigate urea cycle disorders. Clin Chim Acta. 2019;495:406–16.
Bigot A, Tchan MC, Thoreau B, Blasco H, Maillot F. Liver involvement in urea cycle disorders: a review of the literature. J Inherit Metab Dis. 2017;40:757–69.
Todo S, Starzl TE, Tzakis A, Benkov KJ, Kalousek F, Saheki T, et al. Orthotopic liver transplantation for urea cycle enzyme deficiency. Hepatology. 1992;15:419–22.
De Chiara F, Thomsen KL, Habtesion A, Jones H, Davies N, Gracia‐Sancho J, et al. Ammonia scavenging prevents progression of fibrosis in experimental nonalcoholic fatty liver disease. Hepatology. 2020;71:874–92.
Glavind E, Aagaard NK, Grønbæk H, Møller HJ, Orntoft NW, Vilstrup H, et al. Alcoholic hepatitis markedly decreases the capacity for urea synthesis. PLoS One. 2016;11:e0158388.
Petrova PS, Raibekas A, Pevsner J, Vigo N, Anafi M, Moore MK, et al. MANF: a new mesencephalic, astrocyte-derived neurotrophic factor with selectivity for dopaminergic neurons. J Mol Neurosci. 2003;20:173–87.
Yan Y, Rato C, Rohland L, Preissler S, Ron D. MANF antagonizes nucleotide exchange by the endoplasmic reticulum chaperone BiP. Nat Commun. 2019;10:541.
Chen L, Feng L, Wang X, Du J, Chen Y, Yang W, et al. Mesencephalic astrocyte-derived neurotrophic factor is involved in inflammation by negatively regulating the NF-κB pathway. Sci Rep. 2015;5:1–13.
Lindahl M, Danilova T, Palm E, Lindholm P, Võikar V, Hakonen E, et al. MANF is indispensable for the proliferation and survival of pancreatic β cells. Cell Rep. 2014;7:366–75.
Pakarinen E, Danilova T, Võikar V, Chmielarz P, Piepponen P, Airavaara M, et al. MANF Ablation causes prolonged activation of the UPR without neurodegeneration in the mouse midbrain dopamine system. eNeuro. 2020;7:ENEURO.0477-19.2019.
Sousa-Victor P, Jasper H, Neves J. Trophic factors in inflammation and regeneration: the role of MANF and CDNF. Front Physiol. 2018;9:1629.
Neves J, Zhu J, Sousa-Victor P, Konjikusic M, Riley R, Chew S, et al. Immune modulation by MANF promotes tissue repair and regenerative success in the retina. Science. 2016;353:aaf3646.
Hou C, Wang D, Li X, He Y, Wei C, Jiang R, et al. MANF regulates splenic macrophage differentiation in mice. Immunol Lett. 2019;212:37–45.
Jӓntti M, Harvey BK. Trophic activities of endoplasmic reticulum proteins CDNF and MANF. Cell Tissue Res. 2020;382:83–100.
He M, Wang C, Long XH, Peng JJ, Liu DF, Yang GY, et al. Mesencephalic astrocyte-derived neurotrophic factor ameliorates steatosis in HepG2 cells by regulating hepatic lipid metabolism. World J Gastroenterol. 2020;26:1029–41.
Sousa-Victor P, Neves J, Cedron-Craft W, Ventura PB, Liao CY, Riley RR, et al. MANF regulates metabolic and immune homeostasis in ageing and protects against liver damage. Nat Metab. 2019;1:276–90.
Chhetri G, Liang Y, Shao J, Han D, Yang Y, Hou C, et al. Role of mesencephalic astrocyte-derived neurotrophic factor in alcohol-induced liver injury. Oxid Med Cell Longev. 2020;2020:9034864.
Liu J, Wu Z, Han D, Wei C, Liang Y, Jiang T, et al. MANF inhibits liver cancer via SUMOylation-related suppression of NF-kappaB/Snail signaling pathway and epithelial-mesenchymal transition. Hepatology. 2019;71:1262–78.
Bertola A, Mathews S, Ki SH, Wang H, Gao B. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat Protoc. 2013;8:627–37.
Li WC, Ralphs KL, Tosh D. Isolation and culture of adult mouse hepatocytes. Methods Mol Biol. 2010;633:185–96.
Apostolou A, Shen Y, Liang Y, Luo J, Fang S. Armet, a UPR-upregulated protein, inhibits cell proliferation and ER stress-induced cell death. Exp Cell Res. 2008;314:2454–67.
Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5.
Ma J, Chen T, Wu S, Yang C, Bai M, Shu K, et al. iProX: an integrated proteome resource. Nucleic Acids Res. 2019;47:D1211–d7.
Herrera Sanchez MB, Previdi S, Bruno S, Fonsato V, Deregibus MC, Kholia S, et al. Extracellular vesicles from human liver stem cells restore argininosuccinate synthase deficiency. Stem Cell Res Ther. 2017;8:176.
Danilova T, Galli E, Pakarinen E, Palm E, Lindholm P, Saarma M, et al. Mesencephalic astrocyte-derived neurotrophic factor (MANF) is highly expressed in mouse tissues with metabolic function. Front Endocrinol (Lausanne). 2019;10:765.
Yang Y, Wang P, Zhang C, Huang F, Pang G, Wei C, et al. Hepatocyte-derived MANF alleviates hepatic ischaemia-reperfusion injury via regulating endoplasmic reticulum stress-induced apoptosis in mice. Liver Int. 2021;41:623–39.
Wang D, Hou C, Cao Y, Cheng Q, Zhang L, Li H, et al. XBP1 activation enhances MANF expression via binding to endoplasmic reticulum stress response elements within MANF promoter region in hepatitis B. Int J Biochem Cell Biol. 2018;99:140–6.
Link JC, Reue K. Genetic basis for sex differences in obesity and lipid metabolism. Annu Rev Nutr. 2017;37:225–45.
Kono H, Wheeler MD, Rusyn I, Lin M, Seabra V, Rivera CA, et al. Gender differences in early alcohol-induced liver injury: role of CD14, NF-kappaB, and TNF-alpha. Am J Physiol Gastrointest Liver Physiol. 2000;278:G652–61.
Richards AL, Eckhardt M, Krogan NJ. Mass spectrometry-based protein–protein interaction networks for the study of human diseases. Mol Syst Biol. 2021;17:e8792.
Madiraju AK, Alves T, Zhao X, Cline GW, Zhang D, Bhanot S, et al. Argininosuccinate synthetase regulates hepatic AMPK linking protein catabolism and ureagenesis to hepatic lipid metabolism. Proc Natl Acad Sci USA 2016;113:E3423–30.
You M, Arteel GE. Effect of ethanol on lipid metabolism. J Hepatol. 2019;70:237–48.
Preedy VR, Reilly ME, Patel VB, Richardson PJ, Peters TJ. Protein metabolism in alcoholism: effects on specific tissues and the whole body. Nutrition. 1999;15:604–8.
De Chiara F, Heebøll S, Marrone G, Montoliu C, Hamilton-Dutoit S, Ferrandez A, et al. Urea cycle dysregulation in non-alcoholic fatty liver disease. J Hepatol. 2018;69:905–15.
Epstein EE, Labouvie E, McCrady BS, Swingle J, Wern J. Development and validity of drinking pattern classification: binge, episodic, sporadic, and steady drinkers in treatment for alcohol problems. Addict Behav. 2004;29:1745–61.
Singal AK, Kamath PS, Gores GJ, Shah VH. Alcoholic hepatitis: current challenges and future directions. Clin Gastroenterol Hepatol. 2014;12:555–64. quiz e31-2.
Lieber CS. Alcoholic fatty liver: its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol. 2004;34:9–19.
Xu S, Di Z, He Y, Wang R, Ma Y, Sun R, et al. Mesencephalic astrocyte-derived neurotrophic factor (MANF) protects against Aβ toxicity via attenuating Aβ-induced endoplasmic reticulum stress. J Neuroinflammation. 2019;16:35.
Argemi J, Latasa MU, Atkinson SR, Blokhin IO, Massey V, Gue JP, et al. Defective HNF4alpha-dependent gene expression as a driver of hepatocellular failure in alcoholic hepatitis. Nat Commun. 2019;10:3126.
Yu Y, Liu DY, Chen XS, Zhu L, Wan LH. MANF: a novel endoplasmic reticulum stress response protein-the role in neurological and metabolic disorders. Oxid Med Cell Longev. 2021;2021:6467679.
Tang Q, Li Y, He J. MANF: an emerging therapeutic target for metabolic diseases. Trends Endocrinol Metab. 2022;33:236–46.
Ji C. New insights into the pathogenesis of alcohol-induced ER stress and liver diseases. Int J Hepatol. 2014;2014:513787.
Zhang G, Liu Q, Li Y, Huang C, Zhou J, Zhao Y, et al. Mesencephalic astrocyte-derived neurotrophic factor alleviates alcohol induced hepatic steatosis via activating Stat3-mediated autophagy. Biochem Biophys Res Commun. 2021;550:197–203.
Supakul R, Liangpunsakul S. Alcoholic-induced hepatic steatosis-role of ceramide and protein phosphatase 2A. Transl Res. 2011;158:77–81.
Liangpunsakul S, Wou SE, Zeng Y, Ross RA, Jayaram HN, Crabb DW. Effect of ethanol on hydrogen peroxide-induced AMPK phosphorylation. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1173–81.
Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016–23.
Demarquoy J, Fairand A, Gautier C, Vaillant R. Demonstration of argininosuccinate synthetase activity associated with mitochondrial membrane: characterization and hormonal regulation. Mol Cell Biochem. 1994;136:145–55.
Glembotski CC. Functions for the cardiomyokine, MANF, in cardioprotection, hypertrophy and heart failure. J Mol Cell Cardiol. 2011;51:512–7.
Glembotski CC, Thuerauf DJ, Huang C, Vekich JA, Gottlieb RA, Doroudgar S. Mesencephalic astrocyte-derived neurotrophic factor protects the heart from ischemic damage and is selectively secreted upon sarco/endoplasmic reticulum calcium depletion. J Biol Chem. 2012;287:25893–904.
Ye Y, Shibata Y, Yun C, Ron D, Rapoport TA. A membrane protein complex mediates retro-translocation from the ER lumen into the cytosol. Nature. 2004;429:841–7.
Bai M, Vozdek R, Hnízda A, Jiang C, Wang B, Kuchar L, et al. Conserved roles of C. elegans and human MANFs in sulfatide binding and cytoprotection. Nat Commun. 2018;9:1–11.
Yagi T, Asada R, Kanekura K, Eesmaa A, Lindahl M, Saarma M, et al. Neuroplastin modulates anti-inflammatory effects of MANF. iScience. 2020;23:101810.
Norisada J, Hirata Y, Amaya F, Kiuchi K, Oh-hashi K. A sensitive assay for the biosynthesis and secretion of MANF using NanoLuc activity. Biochem Biophys Res Commun. 2014;449:483–9.
Acknowledgements
We sincerely thank Dr. Yi Yang, Department of Pathology, First Affiliated Hospital of Anhui Medical University, and Dr. Goma Chhetri, Department of Pediatrics, University of Pittsburgh, for their technical support and suggestions regarding primary hepatocyte isolation and Gao–Binge model establishment. This work was funded by support programs for J. Shao, including the National Natural Science Foundation of China (82100629), Innovation Program for the Selected Returned Overseas Chinese Scholars in Anhui Province (2020LCX020), Scientific Research Staring Foundation for Young Investigators in Anhui Medical University (XJ201920) and Natural Science Research Project of Anhui Colleges and Universities (KJ2020A0137). This work was funded by support programs for Y. Shen, including the National Natural Science Foundation of China (81973336) and the Joint Fund for Regional Innovation and Development from the National Natural Science Foundation of China (U21A20345). This work was also funded by the National College Student Innovation and Entrepreneurship Training Program (202110366050) for Y. Zhang and Y. Wei.
Author information
Authors and Affiliations
Contributions
HYX and JTS designed the experiments and interpreted the data. HYX, YHJ, SYL XZ, YJS and WW performed the experiments. SW conducted the LC/MS experiment and analyzed the data. YYZ and YJW participated in the animal studies. YXS and JTS conceived and supervised the study and wrote the manuscript. All authors declare no competing financial interests.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Xu, Hy., Jiao, Yh., Li, Sy. et al. Hepatocyte-derived MANF mitigates ethanol-induced liver steatosis in mice via enhancing ASS1 activity and activating AMPK pathway. Acta Pharmacol Sin 44, 157–168 (2023). https://doi.org/10.1038/s41401-022-00920-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-022-00920-8