Abstract
Vascular remodeling contributes to the development of a variety of vascular diseases including hypertension and atherosclerosis. Phenotypic transformation of vascular cells, oxidative stress, inflammation and vascular calcification are closely associated with vascular remodeling. Extracellular vesicles (EVs) are naturally released from almost all types of cells and can be detected in nearly all body fluids including blood and urine. EVs affect vascular oxidative stress, inflammation, calcification, and lipid plaque formation; and thereby impact vascular remodeling in a variety of cardiovascular diseases. EVs may be used as biomarkers for diagnosis and prognosis, and therapeutic strategies for vascular remodeling and cardiovascular diseases. This review includes a comprehensive analysis of the roles of EVs in the vascular remodeling in vascular diseases, and the prospects of EVs in the diagnosis and treatment of vascular diseases.
Similar content being viewed by others
Introduction
Pathological vascular remodeling is a vital pathological process that contributes to the development of a variety of cardiovascular diseases including hypertension, atherosclerosis, aortic aneurysm, and restenosis [1]. Approaches to block pathological vascular remodeling may become promising strategies for the treatment of cardiovascular diseases [2]. Vascular remodeling includes structural and functional abnormalities of the vascular wall, which occur in response to injury, and eventually cause vascular diseases. The remodeling is reflected by changes in medial thickness, luminal diameter, transverse areas, and their ratios [3,4,5]. The process of vascular remodeling is closely associated with complicated cellular and molecular mechanisms such as cell proliferation, migration, apoptosis, extracellular matrix (ECM) synthesis and degradation, vascular oxidation, and inflammation. All of these processes are regulated by a variety of bioactive molecules and hemodynamic stimuli or communications between cells, which trigger abnormal changes in the structure and function of vascular walls [6].
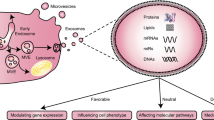
Vesicles in the extracellular space were first described by Peter Wolf in the 1960s [7]; these vesicles were considered inert cellular debris and have been disregarded for decades. Johnstone et al. showed the process of vesicle formation during reticulocyte maturation and coined the term “exosomes” in 1987 [8]. Recently, extracellular vesicles (EVs) have been of concerned important mediators of cell-to-cell communication, and crucial contributors to the physiological regulation and pathogenesis of various diseases [9, 10]. EVs are vesicles that are enclosed by a phospholipid bilayer membrane and contain natural signaling molecules such as proteins, microRNAs (miRNAs), mRNAs and lipids, which are transferred to neighboring or distant cells to modify their phenotype and function [11]. EVs can be released by almost all types of cells. The diameters of EVs are between 30 nm and 5 μm, and EVs can be divided into three types: exosomes (endosomal/intracellular organelle origin), microvesicles (from budding of the cell membrane), and apoptotic bodies (generated during programmed cell death) [12]. The International Society for Extracellular Vesicles recommends using the term “extracellular vesicles” as a collective term for all types of cell-released, membranous vesicles in the extracellular space [13, 14]. EVs play crucial roles in vascular remodeling in vascular diseases such as atherosclerosis, hypertension, pulmonary arterial hypertension, aortic aneurysm, vascular injury and repair [9]. EVs may serve as diagnostic or prognostic biomarkers and therapeutic targets in cardiovascular diseases [15,16,17]. This review summarizes the current research knowledge on EVs in vascular remodeling in cardiovascular diseases and discusses potential biomarkers and therapeutic targets.
Origin of EVs in vascular remodeling
The arterial wall is composed of the intima, media, and adventitia from inside to outside, and the primary cells are vascular endothelial cells (VECs), vascular smooth muscle cells (VSMCs), and vascular adventitial fibroblasts (VAFs), respectively [18]. EVs that contribute to vascular remodeling originate from VECs [19], VSMCs [20], VAFs [21], perivascular adipocytes (PVADs) in perivascular adipose tissue (PVAT) [22], and infiltrated inflammatory cells [12] in the vascular walls. The vessels are also affected by circulating EVs of different cell origins depending on the location of the diseased tissue and the disease states [9] (Fig. 1).
VECs vascular endothelial cells, VSMCs vascular smooth muscle cells, VAFs vascular adventitial fibroblasts, PVADs perivascular adipocytes, ACE angiotensin-converting enzyme, IL-1β interleukin-β, IL-6 interleukin-6, ICAM-1 intercellular adhesion molecule-1, VCAM-1 vascular cell adhesion molecule-1, TNF-α tumor necrosis factor-α, IL-10 interleukin-10, KLF2 Krüppel-like factor-2, Ang II angiotensin II.
EVs from vascular cells
VECs are exposed to the vascular lumen, and are sensitive to shear stress, mechanical injury, chemicals or bioactive molecules. In response to these stimuli, VECs release various signaling molecules, including angiotensin II (Ang II), endothelin, cytokines and growth factors that may modify the phenotype of VECs and VSMCs, and even trigger the development of vascular diseases [23]. EVs from human umbilical vein endothelial cells (HUVECs) transferred miR-143/145 to regulate VSMCs phenotypic target genes in cocultured VSMCs [24]. A recent study showed that EVs from VECs of in the mouse carotid artery regulated the VSMC phenotype [25]. On the other hand, miR-206 inhibited EV release from HUVECs to maintain VSMC contractile phenotype [26]. These findings provide evidence that EV release from VECs contributes to VSMC phenotypic transition.
VSMC-derived EVs from the coronary arteries of 8-month-old domestic crossbred pigs were isolated, and protein profile analyses of EVs showed that the most abundant classes of proteins in microvesicles were cytoplasmic or organelle-associated, housekeeping and metabolic factors, while a high percentage of proteins in exosomes were ECM-related proteins and cell adhesion molecules [20]. Proteomics analysis of human VSMC-derived EVs revealed that the most abundant proteins were related to cell adhesion and platelet activation/coagulation [27]. The miRNA profiles of EVs from the mouse VSMC line MOVAS-1 were modified during calcification [28]. The close link between the VSMC-derived EVs and calcification and coagulation was confirmed in EVs from VSMCs in human aortic tissues [29]. On the other hand, EVs from Ang II-treated human VSMCs resulted in endothelial dysfunction [30]. EVs from VSMCs mediated Krüppel-like factor 5 (KLF5)-induced miR-155 transfer from VSMCs to VECs and damaged the endothelial tight junctions and barrier integrity [31].
VAFs are the most abundant cells in the vascular adventitia, and are important in regulating vascular structure and function [32]. We first isolated and identified EVs from VAFs in the aortas of Wistar-Kyoto rats (WKYs) and spontaneously hypertensive rats (SHRs), and found that EVs from the VAFs of SHRs promoted VSMCs migration [33]. EVs derived from VAFs regulate VSMC proliferation by transferring angiotensin-converting enzyme (ACE), miR-155-5p [21], and miR-135a-5p [34].
EVs from PVADs and infiltrating inflammatory cells
PVAT is the fat tissue surrounding arteries such as the aorta, femoral arteries and small mesenteric arteries [35]. PVAT is considered a metabolically active organ that communicates with other vascular cells by releasing cytokines and chemokines [36, 37]. PVAT can promote VSMC phenotypic transition and vascular remodeling via releasing visfatin, leptin and resistin or attenuate neointimal hyperplasia and vascular remodeling by releasing adiponectin and omentin [38]. Many studies have focused on the roles of EVs derived from the medium of 3T3-L1 cells, a murine adipose cell line, in vascular inflammation [39]. A recent study showed that EVs derived from PVADs from mouse mesenteric adipose tissues could be taken up into VSMCs in vivo and in vitro. EV-mediated miR-221-3p transfer promoted VSMCs proliferation and migration and triggered early-stage vascular remodeling in the context of obesity-associated vascular inflammation [22].
Chronic vascular inflammation plays roles in the development of vascular diseases such as atherosclerosis, hypertension and aortic aneurysm [40]. An excessive inflammatory response, including monocyte/macrophage infiltration, promotes pathological vascular remodeling [3]. Monocyte-derived EVs activate VECs in an interleukin-1β (IL-1β)-dependent manner [41]. Furthermore, EVs from monocytes contribute to the proliferation of VAFs, VSMCs, and VECs [12].
Circulating EVs
Almost all cells can release EVs, but circulating or plasma EVs primarily originate from platelets, red blood cells, leukocytes and vascular cells in the physiological state. Among. cells, platelets and red blood cells greatly outnumber the others [16]. However, some platelet-derived EVs found in the plasma may originate from both platelets and megakaryocytes [42]. Notably, the origin, number and cargo of circulating EVs may change in disease states [43]. Circulating EVs may interact with surface adhesion molecules on VECs, and cause endothelial dysfunction [44]. EVs in serum attenuated muscle damage in a mouse model of acute hindlimb ischemia, possibly through vascular remodeling [45]. Increased circulating EV levels contribute to the development of atherosclerosis by promoting vascular calcification and plaque formation [46].
Evs in vascular inflammation, oxidative stress, and calcification
Chronic vascular inflammation is closely associated with the pathogenesis of vascular remodeling in various vascular diseases, such as hypertension, atherosclerosis and aortic aneurysm [47,48,49]. Studies in humans and animals have shown that vascular inflammation contributes to oxidative stress, while excessive reactive oxygen species (ROS) production induces vascular inflammation [40, 50, 51]. Both inflammation and oxidative stress stimulate the proliferation and migration of vascular cells and thereby promote vascular remodeling [52,53,54]. EVs regulate inflammation, oxidative stress and calcification [39, 55,56,57] and may have an impact on the pathogenesis of vascular diseases.
EVs in vascular inflammation
EVs may contain inflammatory cytokines or anti-inflammatory factors. Yuan et al. found that oxysterol 7-ketocholesterol (7-Ket), an atherogenic stimulator, promoted NLR family pyrin domain-containing 3 (NLRP3) inflammasome formation and activation, inhibited lysosome-multivesicular body fusion, and increased the release of VEC-derived EVs that contained IL-1β. EVs from 7-Ket-stimulated VECs containing IL-1β promoted synthetic phenotypic transition in cocultured VSMCs, while EVs from unstimulated VECs induced the opposite effect [25]. Human activated platelet-derived EVs contain IL-1β and promote the adhesion of neutrophils to human endothelial cells [58]. On the other hand, EVs are able to transfer anti-inflammatory factors. EVs derived from macrophages contained the anti-inflammatory cytokine IL-10, which ameliorates renal tubular injury and inflammation caused by ischemia/reperfusion injury [59].
EVs promote or inhibit inflammation by transferring potent miRNAs and proteins. EVs from mesenchymal stem cells carrying miR-512-3p inhibited oxidized low-density lipoprotein (ox-LDL)-induced VEC proliferation, caspase-3 activation and inflammatory cytokine production [60]. Paeonol, a potential therapeutic drug for atherosclerosis, inhibited NLRP3 inflammasome-mediated inflammation in rat VECs by increasing miRNA-223 levels in the plasma EVs of hyperlipidemic rats [61]. We found that ACE in the EVs derived from VAFs of SHRs promoted VSMC proliferation and migration by increasing Ang II levels in recipient cells [21, 33], which may be involved in promoting inflammation because Ang II is a well-known and potent inflammatory factor.
Fibronectin type III domain-containing 5 (FNDC5) or irisin, a myokine cleaved from FNDC5, attenuated insulin resistance and glucose-lipid metabolic abnormalities in mice with diabetes [62] and inhibited inflammation in the adipose tissues of high-fat-diet-induced obese mice [63]. FNDC5 inhibited ox-LDL-induced foam cell formation and monocyte adhesion in VSMCs by suppressing NF-κB-mediated NLRP3 upregulation [64]. FNDC5 attenuated NLRP3 inflammasome activation and oxidative stress in VSMCs by activating the AMPK-SIRT1 signaling pathway [65] and inhibited VSMC migration and proliferation in SHRs [66, 67]. Increased miR-135a-5p in the EVs from VAFs of SHRs promoted VSMC proliferation by inhibiting FNDC5 expression, which may be involved in the role of FNDC5 in inhibiting inflammation and oxidative stress [34]. EVs from human platelets exerted immunomodulatory effects on VSMCs, and these EVs induced a switch toward a proinflammatory phenotype and promoted VSMC proliferation and migration and vascular remodeling [68].
EVs in vascular oxidative stress
Oxidative stress is involved in EV release, and EVs play a role in promoting oxidative stress. Burger et al. found that Ang II promoted EV release via NADPH oxidase, ROS and Rho kinase targeted to lipid rafts. EVs themselves stimulated endothelial ROS formation and inflammation [69]. Consistently, EVs from oxidative stress-activated VECs promoted VEC proliferation, migration and angiogenesis, which were mediated by miR-92a-3p downregulation in recipient VECs [70]. Ca2+-dependent NADPH oxidase 5 (NOX5) promoted VSMC phenotypic switching and oxidative stress, which increased EV release and subsequent calcification and vascular remodeling [71]. EVs from the VSMCs of chronic kidney disease rats promoted calcification in the recipient VSMCs of normal rats, which were involved in the activation of both NOX and MAPK signaling [72].
It is worth noting that the EVs released under oxidative stress conditions may contain antioxidant molecules that alter the oxidative stress state in target cells and thereby protect against further injury. Nuclear factor erythroid 2-related factor-2 (Nrf2), a redox-sensitive transcription factor, regulates the antioxidant response by increasing the expression of antioxidant enzymes to maintain cellular homeostasis [73]. Nrf2 in EVs regulates the expression of related gene, including antioxidant, anti-inflammatory, cytoprotective and detoxification genes, to modulate oxidative homeostasis in target cells [74].
EVs in vascular calcification
Vascular calcification refers to the abnormal deposition of calcium salts in the vascular wall, resulting in reduced vascular wall elasticity, lumen stenosis and vascular remodeling. Calcification is a cell-mediated process similar to bone mineralization [75] that occurs in vascular aging and is an important pathological process associated with chronic kidney disease, diabetes, arteriosclerosis and hypertension [76]. EVs play important roles in vascular calcification [56] (Fig. 2).
Sustained increases in serum phosphate levels are major contributors to vascular calcification [77, 78]. High levels of phosphate and calcium promote vascular calcification and stimulate osteoblast or chondrocyte differentiation, apoptosis, EV release, and ECM degradation [79]. Chaudhary et al. found that high phosphate levels stimulated EV release from MOVAS cells and altered the protein composition in EVs [80]. High extracellular phosphate levels inhibited the production of calcification inhibitors and resulted in the absence of these inhibitors in the released EVs [81].
The phenotypic transformation of VSMCs into osteogenic cells is necessary for vascular calcification. Bone morphogenetic protein-2 (BMP-2) produced by VECs is a key molecule in the process of osteogenic cell differentiation [82]. Inflammatory factors such as tumor necrosis factor-α (TNF-α) stimulate BMP-2 expression in VECs [83]. Buendía et al. found that EVs from TNF-α-stimulated VECs with high levels of BMP-2 promoted osteogenic differentiation and calcification in VSMCs. Damage to VECs may result in the release of high levels of BMP-2 and calcium and cause osteogenic differentiation in VSMCs and vascular calcification [83]. Furthermore, TNF-α, and platelet-derived growth factor-BB (PDGF-BB) increased EV release from human VSMCs and thereby promoted vascular calcification in response to environmental calcium stress [84].
VSMC phenotypic transformation promoted the EV release from VSMCs under long-term stress and mineral imbalance, and EVs that could calcify and were enriched in the calcified vasculature [84]. The calcified EVs tended to form microcalcifications in areas with sparse collagen when the EVs were released into the ECM, and microcalcification accumulation gradually formed a large calcification area [85]. Sortilin, a multiligand sorting receptor, has the functional characteristics of the vacuolar protein sorting 10 protein (Vps10p) family [86]. Sortilin is a key regulator of VSMC calcification via its recruitment to EVs [87]. Deletion of Sort1, the gene encoding sortilin, reduced the development of atherosclerotic lesions without changing plasma cholesterol levels in a mouse model of atherosclerosis [88].
A variety of miRNAs impact vascular calcification by inhibiting corresponding target gene expression [89,90,91,92]. Chaturvedi et al. performed miRNA microarray analysis to identify the difference in miRNAs between calcifying VSMCs in rat chronic kidney disease and EVs from calcifying VSMCs. The ratio of miRNA to total RNA was increased in EVs compared to VSMCs Fig. 3. There were 33 miRNAs that were differentially expressed between the EVs and VSMCs, and 19 of these miRNAs were downregulated in EVs. The signaling pathways included the MAPK and Wnt signaling pathways, which are important in vascular calcification [93]. Freise et al. isolated EVs from uremic milieu-treated VECs and the plasma of uremic rats and found that uremic EVs augmented VSMC osteogenic transdifferentiation. MiR-221, miR-222, miR-143 and miR-145 were involved in the effects of uremic milieu-treated VECs on vascular calcifications in chronic kidney disease [94]. Furthermore, online hemodiafiltration attenuated inflammation-related VEC dysfunction and vascular calcification in uremic patients by modulating miR-223 expression in plasma EVs [95].
a Roles of EVs from WKY, b roles of EVs from SHR, c roles of EVs-mediated miR-155-5p, ACE, and miR-135a-5p transfer. ACE angiotensin-converting enzyme, Ang II angiotensin II, EVs extracellular vesicles, FNDC5 fibronectin type III domain-containing 5, SHR spontaneously hypertensive rat, VAFs vascular adventitial fibroblasts, VSMCs vascular smooth muscle cells, WKY Wistar-Kyoto rat.
Evs in vascular remodeling in hypertension and atherosclerosis
EVs contribute to the pathological processes of vascular remodeling in a variety of cardiovascular diseases including hypertension and atherosclerosis.
EVs in hypertension
Pathological vascular remodeling is closely associated with the development of hypertension, target organ damage and severe complications [96]. Vascular remodeling in small arteries may be the primary manifestation of target organ damage in hypertension because 100% of patients with stage I hypertension had vascular remodeling in small arteries, while only 60% of patients had endothelial dysfunction and 45% had left ventricular hypertrophy [97]. Hypertensive patients with the highest media:lumen ratios of small arteries had increased incidences of cardiovascular events, suggesting the prognostic importance of small artery structure in hypertension [98]. Arterial stiffening due to vascular remodeling is closely linked to the progression of hypertension and mortality [4]. Antihypertensive agents may affect vascular remodeling directly or indirectly by lowering blood pressure effects [99].
EVs derived from VAFs contribute to vascular remodeling in hypertension [21, 33, 34]. There was no significant difference in the size or number of EVs derived from VAFs from WKYs and SHRs, but EV cargo was changed in SHRs. ACE levels were increased, but miR-155-5p levels were reduced in VAF-derived EVs from SHRs. The EVs from WKYs inhibited VSMC proliferation in SHRs, which was mediated by miR-155-5p in EVs inhibiting ACE transcription (Fig. 4a). EVs from SHRs promoted VSMC migration and proliferation through EV-mediated transfer of ACE and miR-135-5p [21, 33] (Fig. 4b). Overexpression of miR-155-5p attenuated vascular remodeling and hypertension in SHRs [21]. Furthermore, miR-155-5p attenuated VSMC migration in SHRs by inhibiting ACE expression and the downstream Ang II-induced superoxide anion-inflammatory factor pathway [100] (Fig. 4c). These findings suggest that miR-155-5p in EVs inhibit ACE expression, oxidative stress and inflammation in VSMCs from SHRs. Furthermore, dysregulation of miR-135a-5p levels in VSMCs in the pulmonary artery contributed to abnormal VSMC proliferation and the pathogenesis of pulmonary arterial hypertension [101].
The miR-135a-5p level was increased in EVs from the VAFs of SHRs, and the increase in miR-135a-5p in the EVs of SHRs promoted VSMC proliferation by reducing FNDC5 expression, while inhibiting miR-135a-5p prevented the effects of EVs in SHRs on promoting VSMC proliferation [34]. Knockdown of miR-135a-5p in SHRs alleviated vascular remodeling and hypertension [67]. Furthermore, the application of FNDC5 prevented EVs from SHRs in stimulating VSMC proliferation in WKYs and SHRs [34] (Fig. 4c). These findings indicate that both miR-135a-5p and FNDC5 are crucial targets for altering VSMC proliferation and vascular remodeling in hypertension and are supported by the findings that FNDC5 overexpression alleviated vascular remodeling and reduced blood pressure in SHRs [102]. Altering miR-135a-5p or upregulating FNDC5 may be a strategy with some unique advantages in attenuating vascular remodeling and hypertension in obesity, diabetes, atherosclerosis, and other metabolic disorders because of the beneficial effects of FNDC5 on the attenuation of insulin resistance and metabolic disorders.
Knockdown of ACE in the VAFs of SHRs decreased ACE levels in EVs, and the EVs from ACE-knockdown VAFs did not promote VSMC proliferation in WKYs and SHRs [21]. Similarly, knockdown of miR-135a-5p in VAFs prevented the upregulation of miR-135a-5p in EVs. EVs from miR-135a-5p-knockdown did not inhibit FNDC5 expression and promote VSMC proliferation [34]. These results suggest that EV cargo may be affected by the signaling molecule levels in the parent cells.
A recent meaningful study compared the miRNA profiles of plasma EVs from WKYs and SHRs using next-generation sequencing [103]. There were 27 miRNAs that were significantly differentially expressed between WKYs and SHRs, of which 23 miRNAs were upregulated and 4 were downregulated in SHRs. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes pathway analyses were performed for the 1081 candidate target genes of the top 10 differentially expressed miRNAs in the plasma EVs of SHRs. The transforming growth factor-β (TGF-β) and MAPK signaling pathways, which are important signaling pathways associated with blood pressure homeostasis and vascular remodeling [104, 105], were among the top 10 differentially regulated pathways, suggesting that miRNA changes in the circulating EVs of SHRs are involved in vascular remodeling of hypertension.
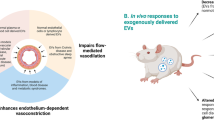
EVs play roles in regulating vascular activity and may be involved in the development of hypertension. Platelet-derived EVs caused thromboxane A2-dependent vasoconstriction [106]. Pfister examined the roles of EVs isolated from the platelet-poor plasma of WKYs and SHRs, as well as normotensive and hypertensive humans. The researchers found that EVs from WKYs reduced acetylcholine (ACh)-induced vasodilation in WKYs but not in SHRs, while EVs from SHRs had no effects on vasodilation in either WKYs or SHRs. Moreover, EVs from normotensive humans inhibited vasodilation in mouse arteries, but no effects of EVs from hypertensive humans were observed. The results indicate that the vasoactive roles of circulating EVs are altered in hypertension [106].
Otani et al. isolated plasma EVs from WKYs and SHRs and performed intraperitoneal injections of EVs weekly for 6 weeks in both strains. The EVs from SHRs increased blood pressure and caused vascular remodeling in WKYs, while the EVs from WKYs attenuated hypertension and vascular remodeling in SHRs [107]. Recently, we isolated EVs from the VAFs of WKYs and SHRs, and intravenous injection of PBS, EVs from WKYs and EVs from SHRs was carried out every 2 days for 10 times. We found that EVs from the VAFs of WKYs attenuated hypertension and vascular remodeling, reduced vascular proliferating cell nuclear antigen (PCNA) and ACE expression, and increased vascular miR-155-5p levels in SHRs. EVs from the VAFs of SHRs increased blood pressure, vascular PCNA and ACE expression, caused vascular remodeling in WKYs, and further deteriorated these changes in SHRs [21]. These findings provide evidence that the application of EVs isolated from plasma or cell culture medium can be used as a strategy to attenuate hypertension, vascular remodeling and vasoactive abnormities.
Multifaceted role of EVs in atherosclerosis
Atherosclerosis is a chronic inflammatory disease that affects blood vessels, triggers plaque formation within the vessel wall and is characterized by endothelial injury, lipid deposition, inflammatory cell infiltration, VSMC phenotypic transformation, foam cell formation, and plaque formation [108]. Atherogenic vascular remodeling involves several cell types, especially VECs and VSMCs. Inflammatory cells such as monocytes and macrophages are recruited to atherosclerotic plaques and are closely associated with the atherosclerosis-associated changes in the vascular wall [109, 110]. EVs can be released from these cells and play important roles in several pathological processes during the initiation and progression of atherosclerosis including inflammation, coagulation, calcification, plaque formation and vascular remodeling [46]. Furthermore, EVs are more abundant and thrombogenic in human atherosclerotic plaques than in plasma [111]. Notably, EVs serve as multifaceted messengers and are able to promote or inhibit atherosclerosis progression [46].
Endothelial dysfunction is a major contributing factor to early atherosclerosis. Infiltration and retention of LDL and the oxidation of LDL to ox-LDL in the intima of arteries initiate a proinflammatory VEC phenotype, endothelial dysfunction, inflammation and atherosclerotic plaque formation [112, 113]. Taguchi et al. found that Ang II and high glucose stimulated EV release from VECs of the aortas in mice, and the resultant EVs aggravated endothelial dysfunction by activating extracellular regulated protein kinase (ERK1/2) signaling and reducing endothelial nitric oxide synthase (eNOS) expression [114]. On the other hand, VEC-derived EVs may exert atheroprotective effects. Hergenreider et al. found that EVs from VECs overexpressing the shear-responsive transcription factor Krüppel-like factor-2 (KLF2) attenuated atherosclerotic lesions in the aortas of ApoE−/− mice [24]. EVs from TNF-α-stimulated HUVECs transferred functional eNOS to protect against lipid-induced endothelial damage and oxidative stress by positively regulating eNOS/Akt signaling [115].
Aberrant lipid metabolism is a crucial event in atheroma formation. EVs play important roles in modulating lipid metabolism including lipid synthesis, transport, and degradation, which are associated with atherosclerosis [116]. Lipids are the main components of EV membranes, and specific lipids are enriched in EVs compared to their parent cells [117]. High-fat diet increased plasma EVs released from VECs in healthy normolipidemic volunteers [118]. Monocyte/platelet-derived EVs activate endothelial cells and promote the formation of human atherosclerotic plaques, suggesting that the attenuation of platelet activation may be a therapeutic strategy for atherosclerosis [119].
Inflammation contributes to the process of atherosclerosis [120], and EVs play a vital role in the proinflammatory response in atherosclerosis. EVs released from infected macrophages stimulated a proinflammatory response in vivo and in vitro [121]. Wu et al. developed a kind of hexyl 5-aminolevulinate hydrochloride (HAL)-engineered M2 macrophage-derived EVs with inflammation-tropism and anti-inflammatory capabilities for atherosclerosis therapy [122]. Dendritic cells are a group of immune cells that act as proinflammatory cells by releasing cytokines, and EVs from mature dendritic cells promote endothelial inflammation and atherosclerosis via the TNF-α-mediated NF-κB pathway [123]. Neutrophils contribute to vascular inflammation and atherogenesis via EV-mediated miR-155 transfer to disease-prone regions [124].
VSMC phenotypic transformation-induced vascular remodeling is a hallmark of atherosclerosis. Platelet-derived EVs induced a switch in VSMCs to a proinflammatory phenotype, promoted VSMC proliferation and migration, and stimulated vascular remodeling [68]. EVs from nicotine-stimulated macrophages accelerated atherosclerosis by increasing VSMC migration and proliferation [125]. Niu et al. found that macrophage foam cell-derived EVs promoted VSMCs migration and adhesion during the progression of atherosclerosis, which may be mediated by the integration of EVs into VSMCs and the subsequent downstream activation of the ERK and Akt pathways in these cells [126]. EVs from circulating leukocytes in atherosclerotic patients were increased compared to those in healthy subjects and promoted the adhesion and migration of VSMCs [127].
EV-loaded miRNAs are involved in atherosclerotic vascular remodeling. Wang et al. found that miR-503-5p was enriched in EVs from ox-LDL-treated macrophages and EVs from the blood mononuclear cells of atherosclerosis patients. EV-mediated miR-503-5p transfer reduced proliferation and angiogenesis in VECs in the human coronary artery but promoted proliferation and migration in VSMCs [128]. An interesting finding was that miR-155 inhibited anti-inflammatory signaling in macrophages, and miR-155 levels in the aorta were decreased during the regression of atherosclerosis in ApoE−/− mice fed a 1% cholesterol diet supplemented with a linoleic acid (CLA) blend but were increased in urinary EVs from patients during atherosclerosis progression [129]. Furthermore, miR-30e and miR-92a were increased in plasma EVs from 42 patients with coronary atherosclerosis [130]. MiRNAs in EVs may be used as biomarkers or intervention targets for atherosclerosis.
Evs as biomarkers in the diagnosis and prognosis of vascular diseases
EVs are found in various body fluids including blood, urine, saliva, bile, breast milk and semen, and dynamically reflect disease states. EVs carry specific cargoes, including proteins, miRNAs, mRNAs, long noncoding RNAs (lncRNAs) and lipids from their parental cells [131]. The cargo in EVs reflects the pathophysiological characteristics of the parent cells or tissues [132, 133]. Furthermore, the lipid bilayer structure protects the genetic materials or specific cargo in EVs from digestion or destruction by circulatory enzymes [134, 135]. EVs have robust potential as biomarkers for vascular remodeling or vascular diseases by integrating EV content and concentrations information [131].
Protein markers in EVs
Some proteins are required for the EV production. Intraluminal vesicles (ILVs) budding into multivesicular endosome (MVE) is mediated by a class of proteins in the endosomal sorting complex required for transport (ESCRT) [136]. The function of the ESCRT pathway depends on its subunit composition and some essential accessory proteins, such as apoptosis-linked gene 2 (ALG-2) interacting protein X (ALIX) and tumor susceptibility gene protein 101 (TSG101) [137]. Exosomes derived from the endolysosomal compartment tend to be enriched in major histocompatibility complex class II (MHC II) and tetraspanins such as CD9, CD63 and CD81. These proteins are closely related to the production of EVs and are generally present in EVs, independent of their cell origin and can be used as markers for the identification of EVs [138].
Some proteins packaged in EVs can serve as biomarkers to reflect the state of a particular disease. Kanhai et al. evaluated the association of some protein levels in plasma microvesicles with the risk of the occurrence of new vascular events in 1060 patients with vascular diseases. The researchers found that cystatin C, serpin F2 and CD14 in plasma microvesicles were closely associated with an increased risk of future vascular events and mortality in patients with vascular diseases [139]. Furthermore, the CD14, serpin G1 and serpin F2 proteins are associated with systemic vascular events in the plasma EVs and correlated with the occurrence of heart failure in 404 patients presenting with dyspnea [140]. Acute coronary syndrome (ACS) is closely related to the vascular changes and lipid metabolism alterations in atherosclerosis [141, 142]. According to a study of 471 frozen serum samples from patients with suspected ACS (30% of whom had an ACS), polygenic immunoglobulin receptor (pIgR), complement factor C5a and cystatin C protein levels in serum EVs were independently associated with ACS [143].
Nucleic acid markers in EVs
Nucleic acids in EVs may be important biomarkers for cardiovascular diseases. EVs contain abundant RNA including mRNA, miRNA, lncRNA, circular RNA (cRNA), small interfering RNA (siRNA), and transfer RNA (tRNA), as well as DNA [138]. The miRNAs loaded in EVs are protected against RNase, exhibit high stability in body fluid [144] and are closely related to vascular remodeling and some vascular diseases [145].
The most interesting miRNAs in EVs that regulate inflammation in association with atherosclerotic disease are the let-7 family, miR-17/92 family, miR-21, miR-29, miR-126, miR-133, miR-146, and miR-155 [146]. Circulating EVs containing miR-199a and miR-126 but not free plasma miRNA predict cardiovascular events in stable coronary artery disease [147]. Gildea et al. examined the miRNAs in urinary EVs and their association with the blood pressure response to dietary salt intake. The researchers found that 45 miRNAs in urinary EVs were associated with the blood pressure response to sodium [148]. The miRNAs in urinary EVs may serve as biomarkers for cardiovascular diseases.
Lipid markers in EVs
Lipids are not only main components of EV membranes, but are also the cargo of EVs. EVs are enriched in sphingomyelin, cholesterol, and phosphatidylserine compared with their membranes of origin [117]. The composition of lipids in EVs may depend on pathophysiological conditions, including cardiovascular diseases with obesity [149]. Ceramides, a type of bioactive sphingolipids, are assembled within the endosome and exacerbate the sorting and production of EVs [150] and may be a valuable biomarker for the risk of cardiovascular events and mortality [151]. Chen et al. performed lipidomics analysis of human plasma EVs and found an abundance of ceramides in EVs [152]. Mannosyl-di-phosphatidylinositol and phosphatidylinositol-ceramides were detected in urinary exosomes and urinary microvesicles, respectively [150]. Changes in lipids in EVs may be potential biomarkers of cardiovascular diseases.
EV counts
EV counts serve as diagnostic or prognostic biomarkers of cardiovascular diseases [9]. Nanoparticle tracking analysis (NTA) is reliable over a range of EVs sizes and concentrations, but the inaccuracy of EV counts is quite high [16], requiring protocols to minimize sample-to-sample variations [153, 154]. Most studies have shown a positive correlation between plasma EV counts and cardiovascular diseases, regardless of the cell type of origin [16]. Kapustin et al. found that the number of exosomes derived from a VSMC calcification model was increased nearly twofold compared with that in the control group, which further promoted the VSMC calcification [9, 84]. Chironi et al. showed that leukocyte-derived circulating microparticles were increased in subclinical atherosclerosis patients, and the amount of microparticles was related to the Framingham risk, preclinical atherosclerosis and metabolic syndrome [155].
Circulating EVs play roles in endothelial damage, platelet activation, hypercoagulability and the regulation of intercellular interactions and are involved in vascular remodeling in atherosclerosis and other vascular diseases [156]. However, plasma EVs are not always positively associated with cardiovascular disease. A recent study showed that there was no significant difference in the concentration or size distribution of plasma EVs between WKYs and SHRs [107]. Furthermore, we showed that the number of EVs derived from the VAFs of SHRs was not greater than that derived from the VAFs of WKYs, but ACE and miR-135a-5p contents were increased in the EVs of SHRs, while miR-155-5p levels were reduced in the EVs of SHRs, contributing to VSMC proliferation, migration and vascular remodeling in hypertension [21, 33, 34, 67].
Circulating EVs can be easily obtained through minimally invasive methods, while urinary EVs can be obtained by a noninvasive method. EVs serve as potential diagnostic or prognostic biomarkers by examining specific changes in the cargo of EVs or the numbers of EVs in cardiovascular diseases, and this method has gained great interest.
Novel therapeutic strategies based on EVs in vascular diseases
Cell therapy research has made enormous progress in the past few decades, but its development has been hampered because of safety issues and the challenges of production. EV therapy is a novel cell-free treatment that is considered one of the most promising therapeutic candidates in the future [9]. The potential of EVs as therapeutic agents benefits from their specific biological characteristics, including biocompatibility, low toxicity, low immunogenicity, biological barrier permeability and relative stability, making them suitable for therapeutic delivery [1]. As mentioned above, EVs inherently contain a variety of natural cargoes. More importantly, exogenous molecules and drugs could be packaged into EVs or engineered EVs due to the breakthroughs in science and technology [11, 157]. A schematic diagram shows the potential therapeutic strategy based on EVs in cardiovascular diseases (Fig. 4).
Direct transfer of isolated EVs
The transfer of EVs isolated from cell medium or body fluids has been shown to have a therapeutic effect on animal models of cardiovascular diseases. Ren et al. found that intravenous administration of EVs from the VAFs of WKYs attenuated hypertension and vascular remodeling in SHRs, whereas EVs from the VAFs of SHRs caused vascular remodeling in WKYs and further deteriorated vascular remodeling in SHRs [21]. Intraperitoneal injection of plasma EVs from SHRs promoted vascular remodeling, while EVs from WKYs attenuated vascular remodeling in SHRs [107].
Brill et al. isolated EVs from healthy human platelet-rich plasma, and then, agarose beads containing EVs were subcutaneously transplanted into the dorsal subcutaneous space of mice. The researchers found that platelet-derived EVs promoted angiogenesis, and the injection of EVs into the ischemic myocardium of mice with chronic ischemia improved revascularization [158]. Recently, Yan et al. isolated EVs from IL-4- and IL-13-treated M2 macrophages and found that M2 macrophage-derived EVs promoted VSMC dedifferentiation and vascular repair after intravascular stent implantation in rats [159]. These findings provide solid evidence that particular EVs can be utilized directly as effective therapeutic agents in animal models of cardiovascular diseases.
Intervention in EV cargo
Altering the parent cells of EVs affects EV characterization and cargo and shows attractive prospects in the treatment of vascular remodeling in vascular diseases. Intervention of parent cells of EVs is one of methods to increase certain beneficial molecule levels or reduce certain adverse molecule levels in EVs for potential treatment of cardiovascular diseases.
Curcumin, a bioactive constituent of curcuma longa, has antioxidant, anti-inflammatory, and anticancer properties [160]. EVs from curcumin-treated mouse brain VECs attenuated oxidative stress and VEC dysfunction during hyperhomocysteinemia [161]. Encapsulation of curcumin in EVs increased the solubility, stability, and bioavailability of curcumin [162].
ACE levels were increased while miR-155-5p contents were reduced in EVs from the VAFs of SHRs, which contributed to EV-mediated promotion of VSMC proliferation and vascular remodeling in SHRs. Knockdown of ACE in VAFs normalized the ACE levels in EVs, attenuated miR-155-5p downregulation in EVs, and abolished EV-mediated promotion of VSMC proliferation in SHRs [21]. Tong et al. found that increased miR-135a-5p in EVs derived from the VAFs of SHRs inhibited FNDC5 expression, and knockdown of miR-135a-5p in VAFs reduced miR-135a-5p in EVs, thereby attenuating EV-mediated stimulation of VSMC proliferation [34]. These findings indicate that altering parent cells to affect EV cargo may be a novel strategy for attenuating vascular remodeling.
Intervention in EVs release
EV counts are closely associated with cardiovascular diseases [9]. Stimulation of beneficial EV release or inhibition of adverse EV release from specific cells may have potential therapeutic roles in cardiovascular diseases. The removal of circulating EVs was first used as a therapeutic strategy for cancers via a hemofiltration system [163]. The system captured circulating EVs originating from cancer cells expressing the marker of human epidermal growth factor receptor 2 (HER-2) based on the finding that HER-2-positive cancers were particularly aggressive [164].
Recently, Liu et al. showed the importance of inhibiting EV release in regulating VSMC phenotype. The researchers found that inhibiting neutral sphingomyelinase (N-SMase) with an N-SMase spiroepoxide inhibitor in HUVECs inhibited EV release and thereby affected the VSMC phenotype [165].
Application of artificial EVs containing specific signaling molecules or drugs
Artificial EVs carrying specific signaling molecules or drugs may be the most critical prospective therapeutic strategy in cardiovascular diseases. Several nanosized carriers have been designed such as liposomes, niosomes, ethosomes, nanoemulsions, transferosomes, dendrimers, polymers, micelles, and solid lipid nanoparticles [166]. These drug delivery systems are nontoxic carriers, have a high drug encapsulation efficiency, and provide sustained release of drugs with good safety, biocompatibility and bioavailability [167]. Some chemical modifications have been made in these carriers to improve their properties such as permeation, penetration and bioavailability. A variety of liposomes with surface modifications of blood-brain barrier-targeting ligands have been created to cross the blood-brain barrier via transcytosis to facilitate the therapeutic efficacy of drugs [168].
Deformable liposomes are bilayer vesicles that are used for drug delivery and have been given different names in previous studies, such as flexible liposomes, ultradeformable liposomes, ultraflexible liposomes and elastic liposomes, and these liposomes are used to transfer drugs for their biochemical, cosmetic and therapeutic purposes. These vesicles exhibit good characteristics in improving the flexibility, penetration, and delivery of drugs to deeper parts of the tissues [169].
Imatinib mesylate, a PDGF-receptor tyrosine kinase inhibitor, is able to attenuate pulmonary arterial hypertension by reversing pulmonary vascular remodeling [170]. Xu et al. developed imatinib mesylate-loaded liposomes and administered them via the pulmonary route to delay drug release and improve patient compliance and treat pulmonary arterial hypertension [171]. Artificial EVs carrying drugs may be a major therapy for vascular remodeling and vascular diseases.
Conclusions and perspectives
EVs are naturally secreted by almost all cells and are present in nearly all body fluids, including blood, urine, saliva and bile. The origin of EVs in the occurrence and progression of vascular remodeling varies and mainly includes vascular cell components, such as VECs, VSMCs, VAFs, PVADs, infiltrated inflammatory cells, and various blood cells such as platelets, red blood cells, and leukocytes. EVs affect vascular oxidative stress, inflammation, calcification, lipid plaque formation and thereby have an impact on vascular remodeling in a variety of cardiovascular diseases including atherosclerosis, hypertension, aortic aneurysm and restenosis. EVs are potential diagnostic and prognostic biomarkers for cardiovascular diseases. EVs have robust application prospects in the treatment of vascular remodeling and cardiovascular diseases.
Although many achievements in EV research have been made in previous studies, there are several shortcomings that need urgent attention in future studies. First, the regulation of EV release is associated with the number and specific cargo of EVs, especially the dysregulation of EVs in particular pathological states or cardiovascular diseases. Further studies will help to reveal crucial targets that regulate EV counts and specific cargo in various cardiovascular diseases. Increasing or reducing endogenous EV release or the levels of specific signaling molecules in EVs may be a promising solution for some diseases.
Second, most of the current studies have been limited to animal experiments, and the safety issues and effective doses of EVs for treatment require extensive in vivo studies and verification. The preparation of amounts of efficient and pure artificial EVs is still a challenge. High purity and large-scale technology to isolate and purify EVs are required to fulfill clinical requirements.
Third, more delicately designed studies are needed to determine or identify out effective biomarkers for particular cardiovascular diseases. EVs and EV-associated RNAs and proteins have significant potential as clinically useful biomarkers for the diagnosis and prognosis of cardiovascular diseases.
Finally, the administration of EVs may be a promising therapeutic strategy in cardiovascular diseases. More in vivo studies focused on EV therapy should be performed in several animal models of cardiovascular diseases for the future development of cardiovascular disease therapies. Moreover, EVs that target special tissues or organs need further investigation to obtain effective EV-targeted therapy. Artificial EVs carrying specific signaling molecules or drugs may have unique advantages in the treatment of cardiovascular diseases.
References
Su SA, Xie Y, Fu Z, Wang Y, Wang JA, Xiang M. Emerging role of exosome-mediated intercellular communication in vascular remodeling. Oncotarget. 2017;8:25700–12.
Wang X, Khalil RA. Matrix metalloproteinases, vascular remodeling, and vascular disease. Adv Pharmacol. 2018;81:241–330.
Anzai T. Inflammatory mechanisms of cardiovascular remodeling. Circ J. 2018;82:629–35.
Humphrey JD. Mechanisms of vascular remodeling in hypertension. Am J Hypertens. 2021;34:432–41.
Montezano AC, Tsiropoulou S, Dulak-Lis M, Harvey A, Camargo LL, Touyz RM. Redox signaling, Nox5 and vascular remodeling in hypertension. Curr Opin Nephrol Hypertens. 2015;24:425–33.
Shi N, Mei X, Chen SY. Smooth muscle cells in vascular remodeling. Arterioscler Thromb Vasc Biol. 2019;39:e247–52.
Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13:269–88.
Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262:9412–20.
Huang C, Neupane YR, Lim XC, Shekhani R, Czarny B, Wacker MG, et al. Extracellular vesicles in cardiovascular disease. Adv Clin Chem. 2021;103:47–95.
Javeed N, Mukhopadhyay D. Exosomes and their role in the micro-/macro-environment: a comprehensive review. J Biomed Res. 2017;31:386–94.
Urabe F, Kosaka N, Ito K, Kimura T, Egawa S, Ochiya T. Extracellular vesicles as biomarkers and therapeutic targets for cancer. Am J Physiol Cell Physiol. 2020;318:C29–39.
Oggero S, ustin-Williams S, Norling LV. The contrasting role of extracellular vesicles in vascular inflammation and tissue repair. Front Pharmacol. 2019;10:1479.
Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750.
Lotvall J, Hill AF, Hochberg F, Buzas EI, Di VD, Gardiner C, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:26913.
Chen Y, Tang Y, Fan GC, Duan DD. Extracellular vesicles as novel biomarkers and pharmaceutic targets of diseases. Acta Pharmacol Sin. 2018;39:499–500.
Dickhout A, Koenen RR. Extracellular vesicles as biomarkers in cardiovascular disease; chances and risks. Front Cardiovasc Med. 2018;5:113.
Zhang JR, Sun HJ. Extracellular vesicle-mediated vascular cell communications in hypertension: mechanism insights and therapeutic potential of ncRNAs. Cardiovasc Drugs Ther. 2020. https://doi.org/10.1007/s10557-020-07080-z.
Mazurek R, Dave JM, Chandran RR, Misra A, Sheikh AQ, Greif DM. Vascular cells in blood vessel wall development and disease. Adv Pharmacol. 2017;78:323–50.
Mendez-Barbero N, Gutierrez-Munoz C, Colio LMB. Cellular crosstalk between endothelial and smooth muscle cells in vascular wall remodeling. Int J Mol Sci. 2021;22:7284.
Comelli L, Rocchiccioli S, Smirni S, Salvetti A, Signore G, Citti L, et al. Characterization of secreted vesicles from vascular smooth muscle cells. Mol Biosyst. 2014;10:1146–52.
Ren XS, Tong Y, Qiu Y, Ye C, Wu N, Xiong XQ, et al. MiR155-5p in adventitial fibroblasts-derived extracellular vesicles inhibits vascular smooth muscle cell proliferation via suppressing angiotensin-converting enzyme expression. J Extracell Vesicles. 2020;9:1698795.
Li X, Ballantyne LL, Yu Y, Funk CD. Perivascular adipose tissue-derived extracellular vesicle miR-221-3p mediates vascular remodeling. FASEB J. 2019;33:12704–22.
Sun HJ, Wu ZY, Nie XW, Bian JS. Role of endothelial dysfunction in cardiovascular diseases: the link between inflammation and hydrogen sulfide. Front Pharmacol. 2019;10:1568.
Hergenreider E, Heydt S, Treguer K, Boettger T, Horrevoets AJ, Zeiher AM, et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012;14:249–56.
Yuan X, Bhat OM, Samidurai A, Das A, Zhang Y, Li PL. Reversal of endothelial extracellular vesicle-induced smooth muscle phenotype transition by hypercholesterolemia stimulation: role of NLRP3 inflammasome activation. Front Cell Dev Biol. 2020;8:597423.
Lin X, He Y, Hou X, Zhang Z, Wang R, Wu Q. Endothelial cells can regulate smooth muscle cells in contractile phenotype through the miR-206/ARF6&NCX1/exosome axis. PLoS One. 2016;11:e0152959.
Qiu H, Shi S, Wang S, Peng H, Ding SJ, Wang L. Proteomic profiling exosomes from vascular smooth muscle cell. Proteom Clin Appl. 2018;12:e1700097.
Pan W, Liang J, Tang H, Fang X, Wang F, Ding Y, et al. Differentially expressed microRNA profiles in exosomes from vascular smooth muscle cells associated with coronary artery calcification. Int J Biochem Cell Biol. 2020;118:105645.
Kapustin AN, Schoppet M, Schurgers LJ, Reynolds JL, McNair R, Heiss A, et al. Prothrombin loading of vascular smooth muscle cell-derived exosomes regulates coagulation and calcification. Arterioscler Thromb Vasc Biol. 2017;37:e22–32.
Song T, Lv M, Zhang L, Zhang X, Song G, Huang M, et al. The protective effects of tripeptides VPP and IPP against small extracellular vesicles from angiotensin ii-induced vascular smooth muscle cells mediating endothelial dysfunction in human umbilical vein endothelial cells. J Agric Food Chem. 2020;68:13730–41.
Zheng B, Yin WN, Suzuki T, Zhang XH, Zhang Y, Song LL, et al. Exosome-mediated miR-155 transfer from smooth muscle cells to endothelial cells induces endothelial injury and promotes atherosclerosis. Mol Ther. 2017;25:1279–94.
Stenmark KR, Nozik-Grayck E, Gerasimovskaya E, Anwar A, Li M, Riddle S, et al. The adventitia: Essential role in pulmonary vascular remodeling. Compr Physiol. 2011;1:141–61.
Tong Y, Ye C, Ren XS, Qiu Y, Zang YH, Xiong XQ, et al. Exosome-mediated transfer of ACE (angiotensin-converting enzyme) from adventitial fibroblasts of spontaneously hypertensive rats promotes vascular smooth muscle cell migration. Hypertension. 2018;72:881–8.
Tong Y, Ye C, Zheng F, Bo JH, Wu LL, Han Y, et al. Extracellular vesicle-mediated miR135a-5p transfer in hypertensive rat contributes to vascular smooth muscle cell proliferation via targeting FNDC5. Vasc Pharmacol. 2021;140:106864.
Samuel OO. Review on multifaceted involvement of perivascular adipose tissue in vascular pathology. Cardiovasc Pathol. 2020;49:107259.
Man AWC, Zhou Y, Xia N, Li H. Perivascular adipose tissue as a target for antioxidant therapy for cardiovascular complications. Antioxidants (Basel). 2020;9:574.
Zhu X, Zhang HW, Chen HN, Deng XJ, Tu YX, Jackson AO, et al. Perivascular adipose tissue dysfunction aggravates adventitial remodeling in obese mini pigs via NLRP3 inflammasome/IL-1 signaling pathway. Acta Pharmacol Sin. 2019;40:46–54.
Zhang YY, Shi YN, Zhu N, Zhao TJ, Guo YJ, Liao DF, et al. PVAT targets VSMCs to regulate vascular remodelling: angel or demon. J Drug Target. 2021;29:467–75.
Connolly KD, Rees DA, James PE. Role of adipocyte-derived extracellular vesicles in vascular inflammation. Free Radic Biol Med. 2021;172:58–64.
Steven S, Frenis K, Oelze M, Kalinovic S, Kuntic M, Bayo Jimenez MT, et al. Vascular inflammation and oxidative stress: major triggers for cardiovascular disease. Oxid Med Cell Longev. 2019;2019:7092151.
Wang JG, Williams JC, Davis BK, Jacobson K, Doerschuk CM, Ting JP, et al. Monocytic microparticles activate endothelial cells in an IL-1β-dependent manner. Blood. 2011;118:2366–74.
Flaumenhaft R, Dilks JR, Richardson J, Alden E, Patel-Hett SR, Battinelli E, et al. Megakaryocyte-derived microparticles: direct visualization and distinction from platelet-derived microparticles. Blood. 2009;113:1112–21.
Shah R, Patel T, Freedman JE. Circulating extracellular vesicles in human disease. N Engl J Med. 2018;379:958–66.
Deng W, Tang T, Hou Y, Zeng Q, Wang Y, Fan W, et al. Extracellular vesicles in atherosclerosis. Clin Chim Acta. 2019;495:109–17.
Cavallari C, Ranghino A, Tapparo M, Cedrino M, Figliolini F, Grange C, et al. Serum-derived extracellular vesicles (EVs) impact on vascular remodeling and prevent muscle damage in acute hind limb ischemia. Sci Rep. 2017;7:8180.
Konkoth A, Saraswat R, Dubrou C, Sabatier F, Leroyer AS, Lacroix R, et al. Multifaceted role of extracellular vesicles in atherosclerosis. Atherosclerosis. 2021;319:121–31.
Siti HN, Kamisah Y, Kamsiah J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vasc Pharmacol. 2015;71:40–56.
Lee YR, Joo HK, Jeon BH. The biological role of apurinic/apyrimidinic endonuclease1/redox factor-1 as a therapeutic target for vascular inflammation and as a serologic biomarker. Biomedicines. 2020;8:57.
Sun HJ, Ren XS, Xiong XQ, Chen YZ, Zhao MX, Wang JJ, et al. NLRP3 inflammasome activation contributes to VSMC phenotypic transformation and proliferation in hypertension. Cell Death Dis. 2017;8:e3074.
Marchio P, Guerra-Ojeda S, Vila JM, Aldasoro M, Victor VM, Mauricio MD. Targeting early atherosclerosis: a focus on oxidative stress and inflammation. Oxid Med Cell Longev. 2019;2019:8563845.
Sturza A, Popoiu CM, Ionica M, Duicu OM, Olariu S, Muntean DM, et al. Monoamine oxidase-related vascular oxidative stress in diseases associated with inflammatory burden. Oxid Med Cell Longev. 2019;2019:8954201.
Garcia-Redondo AB, Aguado A, Briones AM, Salaices M. NADPH oxidases and vascular remodeling in cardiovascular diseases. Pharmacol Res. 2016;114:110–20.
Durgin BG, Straub AC. Redox control of vascular smooth muscle cell function and plasticity. Lab Invest. 2018;98:1254–62.
Sena CM, Leandro A, Azul L, Seica R, Perry G. Vascular oxidative stress: impact and therapeutic approaches. Front Physiol. 2018;9:1668.
Chiaradia E, Tancini B, Emiliani C, Delo F, Pellegrino RM, Tognoloni A, et al. Extracellular vesicles under oxidative stress conditions: biological properties and physiological roles. Cells. 2021;10:1763.
Yang W, Zou B, Hou Y, Yan W, Chen T, Qu S. Extracellular vesicles in vascular calcification. Clin Chim Acta. 2019;499:118–22.
Akbar N, Paget D, Choudhury RP. Extracellular vesicles in innate immune cell programming. Biomedicines. 2021;9:713.
Lindemann S, Tolley ND, Dixon DA, McIntyre TM, Prescott SM, Zimmerman GA, et al. Activated platelets mediate inflammatory signaling by regulated interleukin 1beta synthesis. J Cell Biol. 2001;154:485–90.
Tang TT, Wang B, Wu M, Li ZL, Feng Y, Cao JY, et al. Extracellular vesicle-encapsulated IL-10 as novel nanotherapeutics against ischemic AKI. Sci Adv. 2020;6:eaaz0748.
Chen S, Zhou H, Zhang B, Hu Q. Exosomal miR-512-3p derived from mesenchymal stem cells inhibits oxidized low-density lipoprotein-induced vascular endothelial cells dysfunction via regulating Keap1. J Biochem Mol Toxicol. 2021;35:1–11.
Shi X, Xie X, Sun Y, He H, Huang H, Liu Y, et al. Paeonol inhibits NLRP3 mediated inflammation in rat endothelial cells by elevating hyperlipidemic rats plasma exosomal miRNA-223. Eur J Pharmacol. 2020;885:173473.
Liu TY, Shi CX, Gao R, Sun HJ, Xiong XQ, Ding L, et al. Irisin inhibits hepatic gluconeogenesis and increases glycogen synthesis via the PI3K/Akt pathway in type 2 diabetic mice and hepatocytes. Clin Sci. 2015;129:839–50.
Xiong XQ, Geng Z, Zhou B, Zhang F, Han Y, Zhou YB, et al. FNDC5 attenuates adipose tissue inflammation and insulin resistance via AMPK-mediated macrophage polarization in obesity. Metabolism. 2018;83:31–41.
Zang YH, Chen D, Zhou B, Chen AD, Wang JJ, Gao XY, et al. FNDC5 inhibits foam cell formation and monocyte adhesion in vascular smooth muscle cells via suppressing NFκB-mediated NLRP3 upregulation. Vasc Pharmacol. 2019;121:106579.
Zhou B, Qiu Y, Wu N, Chen AD, Zhou H, Chen Q, et al. FNDC5 attenuates oxidative stress and NLRP3 inflammasome activation in vascular smooth muscle cells via activating the AMPK-SIRT1 signal pathway. Oxid Med Cell Longev. 2020;2020:6384803.
Zhou B, Wu LL, Zheng F, Wu N, Chen AD, Zhou H, et al. miR-31-5p promotes oxidative stress and vascular smooth muscle cell migration in spontaneously hypertensive rats via inhibiting FNDC5 expression. Biomedicines. 2021;9:1009.
Ye C, Tong Y, Wu N, Wan GW, Zheng F, Chen JY, et al. Inhibition of miR-135a-5p attenuates vascular smooth muscle cell proliferation and vascular remodeling in hypertensive rats. Acta Pharmacol Sin. 2021;42:1798–807.
Vajen T, Benedikter BJ, Heinzmann ACA, Vasina EM, Henskens Y, Parsons M, et al. Platelet extracellular vesicles induce a pro-inflammatory smooth muscle cell phenotype. J Extracell Vesicles. 2017;6:1322454.
Burger D, Montezano AC, Nishigaki N, He Y, Carter A, Touyz RM. Endothelial microparticle formation by angiotensin II is mediated via Ang II receptor type I/NADPH oxidase/ Rho kinase pathways targeted to lipid rafts. Arterioscler Thromb Vasc Biol. 2011;31:1898–907.
Li S, Yuan L, Su L, Lian Z, Liu C, Zhang F, et al. Decreased miR-92a-3p expression potentially mediates the pro-angiogenic effects of oxidative stress-activated endothelial cell-derived exosomes by targeting tissue factor. Int J Mol Med. 2020;46:1886–98.
Furmanik M, Chatrou M, van GR, Akbulut A, Willems B, Schmidt H, et al. Reactive oxygen-forming Nox5 links vascular smooth muscle cell phenotypic switching and extracellular vesicle-mediated vascular calcification. Circ Res. 2020;127:911–27.
Chen NX, O’Neill KD, Moe SM. Matrix vesicles induce calcification of recipient vascular smooth muscle cells through multiple signaling pathways. Kidney Int. 2018;93:343–54.
Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–26.
Kahroba H, vatgaran-Taghipour Y. Exosomal Nrf2: From anti-oxidant and anti-inflammation response to wound healing and tissue regeneration in aged-related diseases. Biochimie. 2020;171-172:103–9.
Jiang W, Zhang Z, Li Y, Chen C, Yang H, Lin Q, et al. The cell origin and role of osteoclastogenesis and osteoblastogenesis in vascular calcification. Front Cardiovasc Med. 2021;8:639740.
Hou YC, Lu CL, Yuan TH, Liao MT, Chao CT, Lu KC. The epigenetic landscape of vascular calcification: an integrative perspective. Int J Mol Sci. 2020;21:980
Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87:E10–7.
Abbasian N. Vascular calcification mechanisms: updates and renewed insight into signaling pathways involved in high phosphate-mediated vascular smooth muscle cell calcification. Biomedicines. 2021;9:804.
Houben E, Neradova A, Schurgers LJ, Vervloet M. The influence of phosphate, calcium and magnesium on matrix Gla-protein and vascular calcification: a systematic review. G Ital Nefrol. 2016;33:5.
Chaudhary SC, Khalid S, Smethurst V, Monier D, Mobley J, Huet A, et al. Proteomic profiling of extracellular vesicles released from vascular smooth muscle cells during initiation of phosphate-induced mineralization. Connect Tissue Res. 2018;59:55–61.
Voelkl J, Lang F, Eckardt KU, Amann K, Kuro O, Pasch A, et al. Signaling pathways involved in vascular smooth muscle cell calcification during hyperphosphatemia. Cell Mol Life Sci. 2019;76:2077–91.
Li X, Yang HY, Giachelli CM. BMP-2 promotes phosphate uptake, phenotypic modulation, and calcification of human vascular smooth muscle cells. Atherosclerosis. 2008;199:271–7.
Buendia P, Montes de OA, Madueno JA, Merino A, Martin-Malo A, Aljama P, et al. Endothelial microparticles mediate inflammation-induced vascular calcification. FASEB J. 2015;29:173–81.
Kapustin AN, Chatrou ML, Drozdov I, Zheng Y, Davidson SM, Soong D, et al. Vascular smooth muscle cell calcification is mediated by regulated exosome secretion. Circ Res. 2015;116:1312–23.
Hutcheson JD, Goettsch C, Bertazzo S, Maldonado N, Ruiz JL, Goh W, et al. Genesis and growth of extracellular-vesicle-derived microcalcification in atherosclerotic plaques. Nat Mater. 2016;15:335–43.
Petersen CM, Nielsen MS, Nykjaer A, Jacobsen L, Tommerup N, Rasmussen HH, et al. Molecular identification of a novel candidate sorting receptor purified from human brain by receptor-associated protein affinity chromatography. J Biol Chem. 1997;272:3599–605.
Goettsch C, Hutcheson JD, Aikawa M, Iwata H, Pham T, Nykjaer A, et al. Sortilin mediates vascular calcification via its recruitment into extracellular vesicles. J Clin Invest. 2016;126:1323–36.
Mortensen MB, Kjolby M, Gunnersen S, Larsen JV, Palmfeldt J, Falk E, et al. Targeting sortilin in immune cells reduces proinflammatory cytokines and atherosclerosis. J Clin Invest. 2014;124:5317–22.
He L, Xu J, Bai Y, Zhang H, Zhou W, Cheng M, et al. MicroRNA-103a regulates the calcification of vascular smooth muscle cells by targeting runt-related transcription factor 2 in high phosphorus conditions. Exp Ther Med. 2021;22:1036.
Peng J, Qin C, Tian SY, Peng JQ. MiR-93 inhibits the vascular calcification of chronic renal failure by suppression of Wnt/β-catenin pathway. Int Urol Nephrol. 2022;54:225–35.
Li S, Zhi F, Hu M, Xue X, Mo Y. MiR-133a is a potential target for arterial calcification in patients with end-stage renal disease. Int Urol Nephrol. 2022;54:217–24.
Han R, Luo J, Wang L, Li L, Zheng H. miR-33a-5p suppresses ox-LDL-stimulated calcification of vascular smooth muscle cells by targeting METTL3. Cardiovasc Toxicol. 2021;21:737–46.
Chaturvedi P, Chen NX, O’Neill K, McClintick JN, Moe SM, Janga SC. Differential miRNA expression in cells and matrix vesicles in vascular smooth muscle cells from rats with kidney disease. PLoS One. 2015;10:e0131589.
Freise C, Querfeld U, Ludwig A, Hamm B, Schnorr J, Taupitz M. Uraemic extracellular vesicles augment osteogenic transdifferentiation of vascular smooth muscle cells via enhanced AKT signalling and PiT-1 expression. J Cell Mol Med. 2021;25:5602–14.
Cavallari C, Dellepiane S, Fonsato V, Medica D, Marengo M, Migliori M, et al. Online hemodiafiltration inhibits inflammation-related endothelial dysfunction and vascular calcification of uremic patients modulating miR-223 expression in plasma extracellular vesicles. J Immunol. 2019;202:2372–83.
Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension. 2001;38:581–7.
Park JB, Schiffrin EL. Small artery remodeling is the most prevalent (earliest?) form of target organ damage in mild essential hypertension. J Hypertens. 2001;19:921–30.
Rizzoni D, Porteri E, Boari GE, De CC, Sleiman I, Muiesan ML, et al. Prognostic significance of small-artery structure in hypertension. Circulation. 2003;108:2230–5.
Schiffrin EL. Vascular remodeling in hypertension: mechanisms and treatment. Hypertension. 2012;59:367–74.
Wu N, Ye C, Zheng F, Wan GW, Wu LL, Chen Q, et al. MiR155-5p inhibits cell migration and oxidative stress in vascular smooth muscle cells of spontaneously hypertensive rats. Antioxidants. 2020;9:204.
Liu HM, Jia Y, Zhang YX, Yan J, Liao N, Li XH, et al. Dysregulation of miR-135a-5p promotes the development of rat pulmonary arterial hypertension in vivo and in vitro. Acta Pharmacol Sin. 2019;40:477–85.
Ling L, Chen D, Tong Y, Zang YH, Ren XS, Zhou H, et al. Fibronectin type III domain containing 5 attenuates inflammasome activation and phenotypic transformation of adventitial fibroblasts in spontaneously hypertensive rats. J Hypertens. 2018;36:1104–14.
Liu X, Yuan W, Yang L, Li J, Cai J. miRNA profiling of exosomes from spontaneous hypertensive rats using next-generation sequencing. J Cardiovasc Transl Res. 2019;12:75–83.
Raman M, Cobb MH. TGF-beta regulation by Emilin1: new links in the etiology of hypertension. Cell. 2006;124:893–5.
Liu X, Hu C, Bao M, Li J, Liu X, Tan X, et al. Genome Wide Association Study identifies L3MBTL4 as a novel susceptibility gene for hypertension. Sci Rep. 2016;6:30811.
Pfister SL. Role of platelet microparticles in the production of thromboxane by rabbit pulmonary artery. Hypertension. 2004;43:428–33.
Otani K, Yokoya M, Kodama T, Hori K, Matsumoto K, Okada M, et al. Plasma exosomes regulate systemic blood pressure in rats. Biochem Biophys Res Commun. 2018;503:776–83.
Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–25.
Chistiakov DA, Sobenin IA, Orekhov AN, Bobryshev YV. Human miR-221/222 in physiological and atherosclerotic vascular remodeling. Biomed Res Int. 2015;2015:354517.
Papoutsis K, Kapelouzou A, Georgiopoulos G, Kontogiannis C, Kourek C, Mylonas KS, et al. Tissue-specific relaxin-2 is differentially associated with the presence/size of an arterial aneurysm and the severity of atherosclerotic disease in humans. Acta Pharmacol Sin. 2020;41:745–52.
Leroyer AS, Isobe H, Leseche G, Castier Y, Wassef M, Mallat Z, et al. Cellular origins and thrombogenic activity of microparticles isolated from human atherosclerotic plaques. J Am Coll Cardiol. 2007;49:772–7.
Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95.
Ke LY, Law SH, Mishra VK, Parveen F, Chan HC, Lu YH, et al. Molecular and cellular mechanisms of electronegative lipoproteins in cardiovascular diseases. Biomedicines. 2020;8:550.
Taguchi K, Hida M, Narimatsu H, Matsumoto T, Kobayashi T. Glucose and angiotensin II-derived endothelial extracellular vesicles regulate endothelial dysfunction via ERK1/2 activation. Pflug Arch. 2017;469:293–302.
Mahmoud AM, Wilkinson FL, McCarthy EM, Moreno-Martinez D, Langford-Smith A, Romero M, et al. Endothelial microparticles prevent lipid-induced endothelial damage via Akt/eNOS signaling and reduced oxidative stress. FASEB J. 2017;31:4636–48.
Wang W, Zhu N, Yan T, Shi YN, Chen J, Zhang CJ, et al. The crosstalk: exosomes and lipid metabolism. Cell Commun Signal. 2020;18:119.
Skotland T, Sandvig K, Llorente A. Lipids in exosomes: current knowledge and the way forward. Prog Lipid Res. 2017;66:30–41.
Ferreira AC, Peter AA, Mendez AJ, Jimenez JJ, Mauro LM, Chirinos JA, et al. Postprandial hypertriglyceridemia increases circulating levels of endothelial cell microparticles. Circulation. 2004;110:3599–603.
Oggero S, de GM, Marcone S, Fitzsimons S, Pinto AL, Ikramova D, et al. Extracellular vesicles from monocyte/platelet aggregates modulate human atherosclerotic plaque reactivity. J Extracell Vesicles. 2021;10:12084.
Poznyak AV, Melnichenko AA, Wetzker R, Gerasimova EV, Orekhov AN. NLPR3 inflammasomes and their significance for atherosclerosis. Biomedicines. 2020;8:205.
Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood. 2007;110:3234–44.
Wu G, Zhang J, Zhao Q, Zhuang W, Ding J, Zhang C, et al. Molecularly engineered macrophage-derived exosomes with inflammation tropism and intrinsic heme biosynthesis for atherosclerosis treatment. Angew Chem Int Ed Engl. 2020;59:4068–74.
Gao W, Liu H, Yuan J, Wu C, Huang D, Ma Y, et al. Exosomes derived from mature dendritic cells increase endothelial inflammation and atherosclerosis via membrane TNF-α mediated NF-κB pathway. J Cell Mol Med. 2016;20:2318–27.
Gomez I, Ward B, Souilhol C, Recarti C, Ariaans M, Johnston J, et al. Neutrophil microvesicles drive atherosclerosis by delivering miR-155 to atheroprone endothelium. Nat Commun. 2020;11:214.
Zhu J, Liu B, Wang Z, Wang D, Ni H, Zhang L, et al. Exosomes from nicotine-stimulated macrophages accelerate atherosclerosis through miR-21-3p/PTEN-mediated VSMC migration and proliferation. Theranostics. 2019;9:6901–19.
Niu C, Wang X, Zhao M, Cai T, Liu P, Li J, et al. Macrophage foam cell-derived extracellular vesicles promote vascular smooth muscle cell migration and adhesion. J Am Heart Assoc. 2016;5:e004099.
Vanhaverbeke M, Gal D, Holvoet P. Functional role of cardiovascular exosomes in myocardial injury and atherosclerosis. Adv Exp Med Biol. 2017;998:45–58.
Wang Y, Xu Z, Wang X, Zheng J, Peng L, Zhou Y, et al. Extracellular-vesicle containing miRNA-503-5p released by macrophages contributes to atherosclerosis. Aging. 2021;13:12239–57.
Fitzsimons S, Oggero S, Bruen R, McCarthy C, Strowitzki MJ, Mahon NG, et al. microRNA-155 Is decreased during atherosclerosis regression and is increased in urinary extracellular vesicles during atherosclerosis progression. Front Immunol. 2020;11:576516.
Wang Z, Zhang J, Zhang S, Yan S, Wang Z, Wang C, et al. MiR-30e and miR-92a are related to atherosclerosis by targeting ABCA1. Mol Med Rep. 2019;19:3298–304.
Simeone P, Bologna G, Lanuti P, Pierdomenico L, Guagnano MT, Pieragostino D, et al. Extracellular vesicles as signaling mediators and disease biomarkers across biological barriers. Int J Mol Sci. 2020;21:1694.
Thompson AG, Gray E, Heman-Ackah SM, Mager I, Talbot K, Andaloussi SE, et al. Extracellular vesicles in neurodegenerative disease—pathogenesis to biomarkers. Nat Rev Neurol. 2016;12:346–57.
Roy S, Hochberg FH, Jones PS. Extracellular vesicles: the growth as diagnostics and therapeutics; a survey. J Extracell Vesicles. 2018;7:1438720.
de GA, Geminard C, Fevrier B, Raposo G, Vidal M. Lipid raft-associated protein sorting in exosomes. Blood. 2003;102:4336–44.
Subra C, Laulagnier K, Perret B, Record M. Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie. 2007;89:205–12.
van NG, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–28.
Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14:677–85.
Zaborowski MP, Balaj L, Breakefield XO, Lai CP. Extracellular vesicles: composition, biological relevance, and methods of study. Bioscience. 2015;65:783–97.
Kanhai DA, Visseren FL, Van Der GY, Schoneveld AH, Catanzariti LM, Timmers L, et al. Microvesicle protein levels are associated with increased risk for future vascular events and mortality in patients with clinically manifest vascular disease. Int J Cardiol. 2013;168:2358–63.
Zhang YN, Vernooij F, Ibrahim I, Ooi S, Gijsberts CM, Schoneveld AH, et al. Extracellular vesicle proteins associated with systemic vascular events correlate with heart failure: an observational study in a dyspnoea cohort. PLoS One. 2016;11:e0148073.
Maly M, Hajsl M, Bechynska K, Kucerka O, Sramek M, Suttnar J, et al. Lipidomic analysis to assess oxidative stress in acute coronary syndrome and acute stroke patients. Metabolites. 2021;11:412.
Lunova T, Levytska L, Kucher S, Shatskyi V, Habor H, Klishch I. Observation of serious adverse cardiovascular events over 3 years in patients with advanced atherosclerosis: is there a gender difference? Pol Merkur Lekarski. 2021;49:171–5.
de H, V, Timmers L, Schoneveld AH, Wang JW, van de Weg SM, Sze SK, et al. Serum extracellular vesicle protein levels are associated with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care. 2013;2:53–60.
Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA. 2008;105:10513–8.
Navickas R, Gal D, Laucevicius A, Taparauskaite A, Zdanyte M, Holvoet P. Identifying circulating microRNAs as biomarkers of cardiovascular disease: a systematic review. Cardiovasc Res. 2016;111:322–37.
Hulsmans M, Holvoet P. MicroRNA-containing microvesicles regulating inflammation in association with atherosclerotic disease. Cardiovasc Res. 2013;100:7–18.
Jansen F, Yang X, Proebsting S, Hoelscher M, Przybilla D, Baumann K, et al. MicroRNA expression in circulating microvesicles predicts cardiovascular events in patients with coronary artery disease. J Am Heart Assoc. 2014;3:e001249.
Gildea JJ, Carlson JM, Schoeffel CD, Carey RM, Felder RA. Urinary exosome miRNome analysis and its applications to salt sensitivity of blood pressure. Clin Biochem. 2013;46:1131–4.
Durcin M, Fleury A, Taillebois E, Hilairet G, Krupova Z, Henry C, et al. Characterisation of adipocyte-derived extracellular vesicle subtypes identifies distinct protein and lipid signatures for large and small extracellular vesicles. J Extracell Vesicles. 2017;6:1305677.
Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–7.
Peterson LR, Xanthakis V, Duncan MS, Gross S, Friedrich N, Volzke H, et al. Ceramide remodeling and risk of cardiovascular events and mortality. J Am Heart Assoc. 2018;7:e007931.
Chen S, tta-Chaudhuri A, Deme P, Dickens A, Dastgheyb R, Bhargava P, et al. Lipidomic characterization of extracellular vesicles in human serum. J Circ Biomark. 2019;8:1849454419879848.
Parsons MEM, McParland D, Szklanna PB, Guang MHZ, O’Connell K, O’Connor HD, et al. A protocol for improved precision and increased confidence in nanoparticle tracking analysis concentration measurements between 50 and 120 nm in biological fluids. Front Cardiovasc Med. 2017;4:68.
Vestad B, Llorente A, Neurauter A, Phuyal S, Kierulf B, Kierulf P, et al. Size and concentration analyses of extracellular vesicles by nanoparticle tracking analysis: a variation study. J Extracell Vesicles. 2017;6:1344087.
Chironi G, Simon A, Hugel B, Del PM, Gariepy J, Freyssinet JM, et al. Circulating leukocyte-derived microparticles predict subclinical atherosclerosis burden in asymptomatic subjects. Arterioscler Thromb Vasc Biol. 2006;26:2775–80.
Shantsila E, Kamphuisen PW, Lip GY. Circulating microparticles in cardiovascular disease: implications for atherogenesis and atherothrombosis. J Thromb Haemost. 2010;8:2358–68.
Yin M, Loyer X, Boulanger CM. Extracellular vesicles as new pharmacological targets to treat atherosclerosis. Eur J Pharmacol. 2015;763:90–103.
Brill A, Dashevsky O, Rivo J, Gozal Y, Varon D. Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization. Cardiovasc Res. 2005;67:30–8.
Yan W, Li T, Yin T, Hou Z, Qu K, Wang N, et al. M2 macrophage-derived exosomes promote the c-KIT phenotype of vascular smooth muscle cells during vascular tissue repair after intravascular stent implantation. Theranostics. 2020;10:10712–28.
Han Y, Sun HJ, Tong Y, Chen YZ, Ye C, Qiu Y, et al. Curcumin attenuates migration of vascular smooth muscle cells via inhibiting NFkappaB-mediated NLRP3 expression in spontaneously hypertensive rats. J Nutr Biochem. 2019;72:108212.
Kalani A, Kamat PK, Chaturvedi P, Tyagi SC, Tyagi N. Curcumin-primed exosomes mitigate endothelial cell dysfunction during hyperhomocysteinemia. Life Sci. 2014;107:1–7.
Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18:1606–14.
Marleau AM, Chen CS, Joyce JA, Tullis RH. Exosome removal as a therapeutic adjuvant in cancer. J Transl Med. 2012;10:134.
Ciravolo V, Huber V, Ghedini GC, Venturelli E, Bianchi F, Campiglio M, et al. Potential role of HER2-overexpressing exosomes in countering trastuzumab-based therapy. J Cell Physiol. 2012;227:658–67.
Liu M, Wang D, Gu S, Tian B, Liang J, Suo Q, et al. Micro/nano materials regulate cell morphology and intercellular communication by extracellular vesicles. Acta Biomater. 2021;124:130–8.
Crintea A, Dutu AG, Samasca G, Florian IA, Lupan I, Craciun AM. The nanosystems involved in treating lung cancer. Life. 2021;11:682.
Anita C, Munira M, Mural Q, Shaily L. Topical nanocarriers for management of rheumatoid arthritis: a review. Biomed Pharmacother. 2021;141:111880.
Seo MW, Park TE. Recent advances with liposomes as drug carriers for treatment of neurodegenerative diseases. Biomed Eng Lett. 2021;11:211–6.
Nayak D, Tippavajhala VK. A comprehensive review on preparation, evaluation and applications of deformable liposomes. Iran J Pharmacol Res 2021;20:186–205.
Nakamura K, Akagi S, Ogawa A, Kusano KF, Matsubara H, Miura D, et al. Pro-apoptotic effects of imatinib on PDGF-stimulated pulmonary artery smooth muscle cells from patients with idiopathic pulmonary arterial hypertension. Int J Cardiol. 2012;159:100–6.
Xu H, Ji H, Li Z, Qiao W, Wang C, Tang J. In vivo pharmacokinetics and in vitro release of imatinib mesylate-loaded liposomes for pulmonary delivery. Int J Nanomed. 2021;16:1221–9.
Acknowledgements
The study was supported by National Natural Science Foundation of China (32071106 and 31871148), and Scientific Research and Practice Innovation Program of Jiangsu Province (KYCX20-1376).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Ye, C., Zheng, F., Wu, N. et al. Extracellular vesicles in vascular remodeling. Acta Pharmacol Sin 43, 2191–2201 (2022). https://doi.org/10.1038/s41401-021-00846-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-021-00846-7