Abstract
Hepatocellular carcinoma (HCC), the most prevalent liver cancer, is considered one of the most lethal malignancies with a dismal outcome mainly due to frequent intrahepatic and distant metastasis. In the present study, we demonstrated that oroxylin A, a natural product extracted from Scutellaria radix, significantly inhibits transforming growth factor-beta1 (TGF-β1)-induced epithelial–mesenchymal transition (EMT) and metastasis in HCC. Oroxylin A blocked the TGF-β1/Smad signaling via upregulating the non-steroidal anti-inflammatory drug-activated gene-1 (NAG-1) expression. Oroxylin A promoted NAG-1 transcription by regulating the acetylation of CCAAT/enhancer binding protein β (C/EBPβ), a transcription factor that binds to the NAG-1 promoter. In terms of the underlying mechanism, oroxylin A may interact with histone deacetylase 1 (HDAC1) by forming hydrogen bonds with GLY149 residue and induce proteasome-mediated degradation of HDAC1 subsequently impairing HDAC1-mediated deacetylation of C/EBPβ and promoting the expression of NAG-1. Taken together, our findings revealed a previously unknown tumor-suppressive mechanism of oroxylin A. Oroxylin A should be further investigated as a potential clinical candidate for inhibiting HCC metastasis.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC) is the most common type of liver cancer, mainly occurring in people with chronic liver diseases [1]. The poor prognosis often results from its high recurrence and metastasis after clinical treatments with unclear reasons [2]. As per one of the theories, inadequate surgical removal eventually leads to dissemination or micro-metastasis [3]. With the development of liver carcinogenesis, advanced stage HCCs present a vast molecular heterogeneity resulting from the neoplastic transformation in both epithelial cell-like hepatocytes and progenitor cells. This transformation is mediated by a well-known phenomenon, epithelial–mesenchymal transition (EMT) [4]. Given the complexity of EMT-involved metastasis in HCCs, therapeutics inhibiting HCC metastasis are urgently needed.
The transforming growth factor-beta (TGF-β) pathway mediates a broad spectrum of cellular processes and is implicated in cancer [5]. TGF-β was identified as a critical driver of EMT both during cell development and cancer. Following EMT, transcriptional reprogramming occurs, allowing the cells to acquire the capacity to detach and migrate from the primary tumor [6]. Interestingly, while TGF-β acts as an anti-tumorigenesis factor in the early stages of tumor development, it becomes a pro-tumorigenesis factor in the advanced stages of malignancies [6, 7]. The canonical TGF-β signaling pathway entailing TGF-β-induced activation of receptors usually involves the phosphorylation of Smad2/3. The phosphorylation of Smad2/3 form a complex with Smad4 and gets translocated to the nucleus to trigger or repress the transcription of a series of target genes (Snail family, Zeb family, and bHLH family), resulting in repression of epithelial marker gene expression and activation of mesenchymal gene expression [8]. NAG-1, one of the TGF-β family members, translocates to the nucleus and interferes with the TGF-β1-induced Smad signaling pathway [9]. NAG-1 is often identified as a poor prognostic signature in a series of cancers, and circulating NAG-1 correlates with high rates of metastasis and recurrence [10]. The use of the combination of alpha-feto protein (AFP) and NAG-1 increases the sensitivity of HCC diagnosis [11]. Several reports have highlighted the diverse roles of NAG-1 in cancers. In human hepatocellular carcinoma cell lines, overexpression of NAG-1 has pro-apoptotic and anti-tumorigenic properties [12, 13]. In Apcmin NAG-1 conditional knockout mice, NAG-1 mediates chemoprevention by sulindac in heritable colon cancer [14]. The intricacies of the roles of NAG-1 in cancer progression remain elusive and paradoxical. In this context, the underlying switching mechanism of TGF-β-mediated signaling acquires further investigation.
Oroxylin A (OA, 5,7-dihydroxy-6-methoxyflavone), a natural compound extracted from Scutellaria radix, possesses multiple anti-carcinoma properties–apoptosis induction, differentiation, and overcoming drug resistance in various cells [15, 16]. OA inhibits invasion and metastasis of breast cancer cells and non-small-cell-lung cancer cells [17, 18]. In the present study, we demonstrate that the antineoplastic function of OA in HCC is related to its ability to inhibit cell metastasis in vivo and in vitro, and centered on the ability to induce the expression of NAG-1.
Materials and methods
Reagents and antibodies
OA (purity >99%) and Biotin-OA (purity >99%) were kindly provided by Professor. Zhiyu Li (China Pharmaceutical University, Nanjing, China). OA (purity >99%) and Biotin-OA were dissolved in DMSO (Sigma Aldrich, St. Louis, USA) to prepare a 50 mM stock solution, which was stored at −20 °C. Human recombinant TGF-β1 was purchased from MedChemExpress (Shanghai, China) and dissolved in sterile water. Cycloheximide (CHX) and MG132 were purchased from MedChemExpress (Shanghai, China) and dissolved in DMSO. Antibodies for Smad2/3 (#8685), phospho-Smad2 (Ser465/467)/Smad3 (Ser423/425) (#8828), E-cadherin (#14472), phospho-C/EBPβ (Thr235) (#3084), HDAC1 (#5356), acetylated-lysine (#9441), Lamin A/C (#4777), α/β-Tubulin (#2148), and p300 (#86377) were purchased from Cell Signaling Technology (Beverly, MA, USA). Antibodies for NAG-1 (#sc377195), C/EBPβ (#sc7962), GAPDH (#sc32233), and β-Actin (#sc47778) were purchased from Santa Cruz Biotechnology (Santa cruz, CA, USA). Antibodies for Vimentin (#P08670), N-cadherin (#P199022) were purchased from Bioworld Technology (St.Louis Park, MN, USA). Antibody for Twist1 (#A15596) was purchased from Abclonal (Wuhan, China). Antibodies for c-Myc Epitope Tag (#MA1980) and Alexa FluorTM 488 phalloidin (#A12379) were purchased from Thermo Fisher Scientific (USA). Antibody for GFP-Tag (#M2004) was purchased from AbMART (Shanghai, China).
Plasmid construction and mutagenesis
The SBE4 luciferase reporter gene plasmids (SBE4-Luc), pLkO.1 hygro vectors, pMD2G, psPAX2, pRL-TK, and pLenti-GFP-puro were purchased from Addgene. pGL3-C/EBPβ was purchased from Promega. The TGF-β-inducible reporter construct (p3TP-Lux) was a gift from Joan Massague & Jeff Wrana. pLKO shNAG-1 plasmid was constructed according to the manufacturer’s instructions. We amplified HDAC1 (codons 88-1536) from a cDNA library prepared from HEK 293 T RNA. This fragment was ligated into the pGEX-6P-1 and pLenti-GFP-vectors. Site-directed mutagenesis was carried out using the Mut Express®II Fast Mutagenesis Kit V2 (Vazyme Biotec, Nanjing, China). All the constructs were confirmed by sequencing. Detail primer sequences are given in Supplementary Table 1.
RNA isolation and real-time polymerase chain reaction
Total RNA was isolated from the cells using RNAiso plus reagent (Takara Biomedical Technology, Dalian, China). 1 μg of RNA was reverse-transcribed using HiScript II Q RT SuperMix (Vazyme Biotech, Nanjing, China) according to the manufacturer’s instructions. Real-time PCR was carried out using ChamQ SYBR qPCR master mix (Vazyme Biotech, Nanjing, China). The primer sequences are given in Supplementary Table 2.
Chromatin immunoprecipitation assay
After treatment with OA or vehicle, Chromatin immunoprecipitation (CHIP) assay was carried out using EZ-Magna CHIPTM A/G chromatin immunoprecipitation kit (Merck & Millipore, Massachusetts, USA) according to the manufacturer’s instructions. The detailed primer sequences are given in Supplementary Table 2.
Statistical analysis
Data were presented as mean ± standard deviation (SD). Statistical analysis was performed as described in each figure legend, and sample sizes were shown in each corresponding figure legend. P < 0.05 was considered statistically significant.
Detailed descriptions of all methods are provided in Supplementary materials and methods.
Results
Oroxylin A inhibits TGF-β1-induced migration and invasion of HCC cells
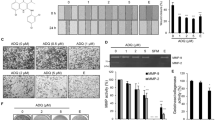
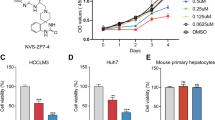
Although the previous studies reported OA-mediated inhibition of migration in breast cancer and lung cancer cells [17, 18], we explored OA’s anti-metastatic effect in HCC cells. Since TGF-β1 is an inducer of EMT and is vital for tumor metastasis [8], we pretreated the cells with TGF-β1 for 12 h, followed by incubation with OA (50 μM) for a total of 48 h. OA remarkably suppressed TGF-β1-induced migration and invasion of HCC cells (Figs. 1a–1d, Supplementary Figs. 1a–1d). Moreover, OA did not induce significant cytotoxicity (Fig. 1e). After 48 h treatment, OA (50 μM) induced apoptosis in SMMC-7721 cells, but the apoptosis rate was lower than 10% (Fig. 1f, g). These observations demonstrate that OA significantly inhibits TGF-β1 induced migration and invasion of HCC cells.
a Wound healing assay. SMMC-7721 cells were treated with TGF-β1 (10 ng/mL) for 12 h, followed by co-incubation with TGF-β1 OA(50μM) or DMSO for a total of 48 h. Representative images photographed at 0 h and 48 h. Scale bar: 100 μm. b Quantification of the wound healing. c After SMMC-7721 cells were treated with OA (50 μM) or DMSO in the presence or absence of TGF-β1 (10 ng/mL) for a total of 48 h and Matrigel-based transwell assay was used to evaluate their invasive properties. Scale bar: 50 μm. d Quantification of the cell invasion rates. e SMMC-7721 cells were treated with OA (0, 6.25, 12.5, 25, 50 μM) for 48 h and CCK8 assay was conducted to evaluate the viability. f Cells were treated with different concentrations of OA for 48 h, and flow cytometry was used to evaluated cells co-stained with Annexin V and PI. Ann.V−/PI− as healthy cells, Ann.V+/PI− as early apoptotic cells, Ann.V+/PI+ probably as late apoptotic cells. g Quantification of apoptosis cell ratio. These data are represented as mean ± SD from triplicate experiments. *P < 0.05 and ***P < 0.001 compared with normal group or referred group. #P < 0.05, ###P < 0.001 compared with TGF-β1 group. n.s. not significant. Differences are tested using two-way ANOVA
Oroxylin A represses TGF-β1-induced EMT in HCC cells
EMT as a pivotal step that confers carcinoma cells with invasive and metastatic properties [19]. TGF-β1 is widely used as an EMT inducer that induces loss of cell polarity, decreases cell adhesion, and increases migration. Here, we investigated the role of OA in TGF-β1-induced EMT in HCC cells, TGF-β1 induced an elongated, fibroblast-like phenotype in HCC cells, which was attenuated by OA treatment (Fig. 2a). Moreover, the distribution of filamentous actin (F-actin) was visualized by phalloidin staining, the data revealed that cytoskeletal rearrangement in the TGF-β1-treated HCC cells was significantly blocked by OA treatment (Fig. 2b and Supplementary Fig. 2a). We also analyzed the expression patterns of EMT-related proteins and found that E-cadherin levels deceased after TGF-β1 treatment, while those of N-cadherin, Vimentin, and Twist1 increased. OA treatment repressed TGF-β1-induced changes in expression patterns of various EMT markers (Fig. 2c, Supplementary Fig. 2b, c). Also, MMP2, and MMP9 are responsible for tumor invasion and metastasis. As per the qPCR results, TGF-β1 decreased the mRNA levels of E-cadherin and increased the mRNA levels of TWIST1, MMP2 and MMP9. These manifestations were reversed by OA treatment (Fig. 2d). These findings suggest that OA suppresses TGF-β1 induced EMT in HCC cells.
a SMMC-7721 cells were treated with OA (50 μM) or DMSO in the presence of TGF-β1 (10 ng/mL)for 48 h and representative photographs were used to display morphology of cells. Scale bar: 20 μm. b Immunofluorescence assay was performed to detect the structure of F-actin with phalloidin staining. Scale bar: 5 μm. c SMMC-7721 cells were treated with OA as indicated doses in the presence or absence of TGF-β1 (10 ng/mL) for 48 h, and Western blot assay was used to evaluate expression level of E-cadherin, N-cadherin, Vimentin, and Twist1. d SMMC-7721 cells were treated with OA (50 μM) or DMSO and co-incubated with TGF-β1 (10 ng/mL) or not, mRNA levels of E-cadherin, TWIST1, MMP2, and MMP9 were measured through RT-PCR. Fold change is represented as the mean ± SD for three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001. Differences are tested using one-way ANOVA
Oroxylin A inhibits the TGF-β1/Smad pathway without affecting Smad2/3 phosphorylation
Since OA was found to suppress TGF-β1-induced EMT in HCC cells, we investigated the role of OA in TGF-β1/Smad mediated transcriptional responses. The expression levels of PAI-1, a target gene of the TGF-β1 signaling pathway, were effectively reduced in OA-treated HCC cell lines (Fig. 3a) [20]. Furthermore, we used p3TP-Lux containing three consecutive TGF-β response elements, and SBE4-Luc containing four copies of the Smad binding sites, to examine the effect of OA on the TGF-β signaling pathway [21]. OA treatment significantly inhibited TGF-β signaling activity (Fig. 3b, c). TGF-β triggers EMT accompanied by Smad2/3 phosphorylation and Smad complex nuclear translocation [22]. We found that OA failed to impact TGF-β1 induced Smad2/3 phosphorylation and Smad complex nuclear translocation (Fig. 3d–f). Taken together, OA inhibits the TGF-β1/Smad pathway without affecting Smad2/3 phosphorylation and Smad complex nuclear translocation.
a SMMC-7721 cells were treated with OA (50 μM) or DMSO and co-incubated with TGF-β1 (10 ng/mL) or not, mRNA levels of PAI-1 were detected by RT-PCR. b p3TP-lux (1 μg) and (c) SBE4-Luc (1 μg) were co-transfected with pRL-TK vectors (0.1 μg) into SMMC-7721 cells, respectively. After 24 h, cells were treated with OA (0, 12.5, 25, 50 μM) for 6 h and TGF-β1 (10 ng/mL) for another 18 h as indicated, followed by luciferase reporter assay. d, e SMMC-7721 cells were treated with TGF-β1 (10 ng/mL) and OA simultaneously for indicated hours or at indicated OA concentrations. The lysates were subjected to Western blot analysis for pSmad2/3 and Smad2/3 and GAPDH was used as a loading control. f SMMC-7721 cells were treated with OA (50 μM) or DMSO for 24 h and incubated with TGF-β1 (10 ng/mL) in serum-free medium for 1 h. Immunofluorescence was performed with Smad2/3 antibody as described in Materials and methods. Scale bar: 20 μm. Data are represented as mean ± SD for three independent experiments. *P < 0.05, ***P < 0.001 compared with normal group or referred group. #P < 0.05, ##P < 0.01, ###P < 0.001 compared with TGF-β1 group. n.s. not significant. Differences are tested using one-way ANOVA
Oroxylin A inhibits the TGF-β1/Smad pathway through upregulating NAG-1 expression
NAG-1, a TGF-β superfamily protein, translocates to the nucleus and contributes to the inhibition of the Smad pathway by interrupting the Smad complex.[9] We found that OA treatment significantly induces NAG-1 expression in the cytoplasm and nucleus of HCC cells (Fig. 4a, b and Supplementary Fig. 3a). To further explore the role of NAG-1 in OA-mediated inhibition of the TGF-β1/Smad pathway, we established a stable NAG-1 knockdown SMMC-7721 cell line. Knock-down of NAG-1 (shNAG-1) abolished OA mediated inhibition of TGF-β1/Smad pathway, as evidenced by the change in expression of E-cadherin and Twist1 after TGF-β1 treatment (Fig. 4c). We analyzed the transcription factor activity of the Smad complex using SBE4-Luc. We found that NAG-1 knock-down abolished OA mediated inhibition of Smad transcription factor activity and the mRNA levels of MMP2 and MMP9, induced by TGF-β1 treatment (Fig. 4d, e). Meanwhile, OA has a limited inhibitory effect on TGF-β1 induced cell invasion in stable NAG-1 knock-down SMMC-7721 cells (Fig. 4f). Further, we overexpressed NAG-1 in SMMC-7721 cells (Supplementary Fig. 3b). The mRNA levels of MMP9 in cells overexpressing NAG-1 were downregulated, and the mRNA levels of E-cadherin were upregulated (Supplementary Fig. 3c). Furthermore, ectopic expression of NAG-1 weakened cell motility and decreased the response of cells to TGF-β1 (Supplementary Fig. 3d). But, OA showed modest anti-metastasis efficacy in the NAG-1 overexpression group after TGF-β1 treatment. The underlying reason may be that the ectopic exorbitant expression of NAG-1 attenuated the anti-metastasis capacity of OA at the treatment dose. These results suggested that the induction of NAG-1 expression mediates OA-induced inhibition of metastasis in HCC.
a SMMC-7721 cells were treated with OA (0, 12.5, 25, 50 μM) for 24 h and the protein level of NAG-1 was evaluated by Western blot and β-Actin was used as a loading control. b SMMC-7721 cells were treated with OA (0, 12.5, 25, 50 μM) for 24 h, nuclear and cytoplasmic protein were extracted, and Western blot analysis of NAG-1. Lamin A/C and α/β-Tubulin were used as controls for nuclear and cytoplasmic fraction, respectively. c The efficiency of NAG-1 stably knock down in SMMC-7721 cells was evaluated by Western blot assay, and EMT-related protein E-cadherin and Twist1 levels were detected. d Luciferase reporter assay of the SBE4 reporter in SMMC-7721 cells transfected with shCon and shNAG-1 were treated with OA (50 μM) or DMSO in the presence or absence of TGF-β1 (10 ng/mL) for 24 h. e qPCR analysis of MMP2 and MMP9 levels in cells stably transfected with shCon or shNAG-1 as indicated treatment. f shCon cells and shNAG−1 cells were treated with OA (50 μM) or DMSO in the presence or absence of TGF-β1 (10 ng/mL) for 48 h. The invasive ability was evaluated by Matrigel-based transwell invasion assay. Scale Bar: 50 μm. And quantification of invasion rates. Data are represented as mean ± SD for three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 compared with normal group or referred group. #P < 0.05, ##P < 0.01, ###P < 0.001 compared with shNAG-1 group. n.s. not significant. Differences are tested using two-way ANOVA
Oroxylin A activates NAG-1 transcription through the C/EBPβ binding motif
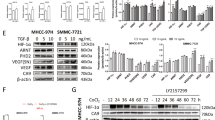
To further explore the underlying mechanism of OA-mediated NAG-1 upregulation, we evaluated the mRNA levels of NAG-1 in SMMC-7721 cells after OA treatment. OA upregulated NAG-1 mRNA expression in a time- and dose-dependent manner (Fig. 5a, b). We further investigated the role of OA in the transcriptional regulation of NAG-1 using a shorter upstream region (−133 to +41 relative to TSS) and a longer region (−1086 to +41 relative to TSS) ahead of the reporter gene. Similar results were obtained from the shorter and longer promoters of NAG-1. We found that the potential transcription factor binding motif involved in this process was located most proximally to the TSS (−133 to +41) (Fig. 5c). Several potential motifs, including C/EBPβ, Sp1, XF1, and RARα, are located in the region (−133 to +41). Some of these motifs play essential roles in NAG-1 transcription (Supplementary Fig. 4a). Moreover, OA promotes NAG-1 transcription in wild-type (WT) promoter or promoters with mutations in Sp1, XF1, and RARα binding sites, except for promoters with C/EBPβ binding site mutation in SMMC-7721 cells, suggesting that C/EBPβ motif may be involved in OA mediated NAG-1 transcription (Fig. 5d). Finally, OA promoted the activity of the C/EBPβ transcription factor (Fig. 5e). Together, these data suggest that OA may activate NAG-1 transcription by regulating the C/EBPβ transcription factor activity.
a, b SMMC-7721 cells were incubated with OA dose- or time-dependently as indicated. The relative NAG-1 mRNA level was measured by qPCR, GAPDH as an internal control. c SMMC-7721 cells were transfected with a reporter gene containing different lengths of NAG-1 promoter and treated with OA (50 μM) or DMSO for 24 h, followed by luciferase activity assay. d SMMC-7721 cells were transfected with each internal mutation construct of NAG-1 promoter (pNAG-1-133/+41) and then treated with OA (50 μM) or DMSO for 24 h, followed by luciferase activity assay. e SMMC-7721 cells were transiently transfected with luciferase reporter of C/EBPβ, then exposed to OA (0, 12.5, 25, 50 μM) for 24 h followed by luciferase activity assay. These data are represented as mean ± SD for three independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with DMSO group. n.s. not significant. Differences are tested using one-way ANOVA
Oroxylin A promotes acetylation of C/EBPβ
Since C/EBPβ plays an essential role in OA-mediated NAG-1 transcription, we investigated the role of OA in regulating the C/EBPβ expression and phosphorylation. Firstly, OA was not found to affect C/EBPβ expression and phosphorylation (Fig. 6a). Secondly, OA did not affect the nuclear translocation of C/EBPβ (Fig. 6b). However, the CHIP assay showed that OA treatment increased the binding of C/EBPβ to NAG-1 promoter, indicating that OA treatment might affect the posttranslational modification of C/EBPβ besides phosphorylation, which is vital for the DNA binding affinity of C/EBPβ (Fig. 6c). The acetylation of C/EBPβ facilitates its binding to the DNA, thereby stimulating C/EBPβ-dependent transcription [23]. Here, we showed that treatment with OA significantly improves the acetylation level of C/EBPβ (Fig. 6d).
a SMMC-7721 cells were incubated with OA (0, 12.5, 25, 50, 100 μM) for 24 h, the protein level of C/EBPβ and pC/EBPβ were analyzed by Western blot. b Immunofluorescence staining of C/EBPβ in SMMC-7721 cells treated with OA (50 μM) or DMSO for 24 h. Scale bar: 20 μm. c Chromatin immunoprecipitation assay was performed to test in vivo binding of C/EBPβ to NAG-1 promoter in SMMC-7721 cells. Cells were treated with OA (50 μM) or DMSO for 24 h as described in Materials and Methods. Normal mouse IgG as the negative control. The results are representative of three independent experiments and expressed as mean ± SD. ***P < 0.001 compared with DMSO group. Differences are tested using Student’s t-test. d SMMC-7721 cells were treated with OA (0, 12.5, 25, 50 μM) for 24 h, and the cell lysate was IP with anti-C/EBPβ. Western blot was performed for acetyl-lysine and C/EBPβ. e Left panel: SMMC-7721 cells were transfected with pCMV-p300-Myc for 24 h, followed by treatment with OA (50 μM) or DMSO for 24 h. Western blot was used for analyzing the exogenous protein level of Myc-p300. Right panel: SMMC-7721 cells were directly treated with OA (50 μM) or DMSO for 24 h to evaluate the endogenous protein level of p300 by Western blot. f Western blot analysis of HDAC1 in SMMC-7721 cells treated with OA (0, 12.5, 25, 50, 100 μM) for 24 h and GAPDH as a loading control. g SMMC-7721 cells were treated with OA (0, 12.5, 25, 50, 100 μM) for 24 h, mRNA levels of HDAC1 were measured through qPCR. Fold change is represented as the mean ± SD for three independent experiments, n.s. not significant. Differences are tested using Student’s t-test. h Western blot analysis of HDAC1 in SMMC-7721 cells treated with OA (50 μM) or DMSO and then with CHX (100 mg/mL) for a total 24 h. Quantification of band intensity is on the right. i Western blot analysis of HDAC1 in SMMC-7721 cells treated with OA (50 μM) or DMSO for 24 h. MG132 (10 μM) was added 4 h before harvesting
The dynamic regulation of the acetylation state of C/EBPβ relies on acetyltransferase and deacetylase. Nuclear coactivator p300 with intrinsic acetyltransferase activity modify multiple lysine sites of C/EBPβ, these modifications are essential for the function of C/EBPβ transcriptional factor [24]. However, treatment of SMMC-7721 cells with OA does not affect exogenous and endogenous levels of p300 protein (Fig. 6e). In SMMC-7721 cells, OA significantly decreased the levels of HDAC1 protein, a modulator of C/EBPβ acetylation (Fig. 6f). These observations suggest that OA can facilitate C/EBPβ acetylation by inhibiting the HDAC1 expression. Next, we found no significant changes in the HDAC1 mRNA levels after OA treatment, suggesting that OA regulated HDAC1 expression at posttranslational level (Fig. 6g). To directly investigate whether OA affects HDAC1 protein stability, we blocked the de novo synthesis of HDAC1 protein using cycloheximide (CHX) and examined HDAC1 degradation. OA reduced the HDAC1 protein stability by decreasing the half-life of HDAC1 (Fig. 6h). Moreover, OA-induced reduction of HDAC1 was attenuated after treatment with a proteasome inhibitor, MG132 (Fig. 6i). Overall, these findings suggest that OA facilitates proteasome-mediated degradation of HDAC1 and promotes C/EBPβ acetylation.
Gly149 is essential for the binding of HDAC1 with oroxylin A
To further explore the mechanism underlying the attenuation of HDAC1 protein expression by OA, we investigated the binding between OA and the crystal structure of HDAC1 [PDB ID:5ICN] using molecular docking techniques. We found several critical directional interactions between the OA scaffold and the HDAC1, a hydrogen bond between the oxygen atom of the carbonyl group and Gly149, a second hydrogen bond between 5-hydroxyl and Asn95. The 2-aryl ring of OA is sandwiched between the hydrophobic residues (mainly Phe150 and Phe205) in a lipophilic region. The Pi-anion interaction between the OA scaffold and the carboxyl of Asp99 further anchors the ligand (OA) in the pocket (Fig. 7a). The proximity of Asp99, Gly149, Phe205, and Phe150 to the HDAC1 active site increases the activity of HDAC1 [25]. To analyze the critical amino acids of HDAC1 involved in interactions with OA, we induced an alanine mutation and extracted the GST-fusion protein (Fig. 7b). We also conducted pull-down assays with biotin-OA and observed that Gly149 was required to bind with OA (Fig. 7c). Further, we performed MST assay to evaluate the dissociation kinetics of OA. OA showed a 4-fold lower binding affinity to HDAC1-G149A (Kd = 208.56 μM) than wild-type HDAC1 (Kd = 48.9 μM) (Fig. 7d). We compared the stability of wild-type HDAC1 and HDAC1-G149A mutant proteins following OA treatment. Treatment of transfected SMMC-7721 cells with OA reduced the GFP-HDAC1-WT protein levels in a dose-dependent manner; treatment with 100 μM OA reduced the GFP-HDAC1-G149A mutant protein levels (Fig. 7e). Further, SMMC-7721 cells transfected with HDAC1-WT/HDAC1-G149A in the presence of CHX (100 μg/mL) to block the newly protein synthesis and examine the degradation of HDAC1-WT/HDAC1-G149A protein levels after OA treatment. HDAC1-WT protein degraded more rapidly than HDAC1-G149A after OA treatment, suggesting that Gly149 mutation weakened the interactions between HDAC1 and OA; Gly149 played a role in OA-mediated HDAC1 degradation (Fig. 7f).
a Chemical structure of OA. Molecular docking analysis of the potential binding between OA and HDAC1. b Coomassie stain of the GST protein and the mutant/wild type GST-HDAC1 proteins. c Pulldown assay to assessOA′s association with HDAC1 wild-type or alanine mutants. Arrows and asterisks mark specific and nonspecific bands, respectively. d Dose–response curve for the binding interaction between OA and HDAC1-WT or HDAC1-G149A lysate. The concentration of HDAC1-FITC protein is kept constant at 20 nM, while the OA concentration varies from 15 nM to 0.5 mM. The results are representative of three independent experiments and expressed as mean ± SD. e Comparison of exogenous HDAC1-WT and HDAC1-G149A expressions in SMMC-7721 cells treated with OA (0, 12.5, 25, 50, 100 μM) for 24 h. f Western blot analysis of exogenous expression of HDAC1-WT and HDAC1-G149A in SMMC-7721 cells treated with DMSO or OA (50 μM) and then with CHX (100 μg/mL) for a total of 24 h. And the quantification of band intensity. g Schematic diagram of Oroxylin A inhibits HCC metastasis via inducing NAG-1 expression
Oroxylin A reduces pulmonary metastasis of SMMC-7721 cells in vivo
We established a lung metastasis model using 5-week-old female BALB/c nude mice to investigate the anti-metastasis ability of OA and NAG-1’s role in vivo. OA treatment reduced lung mass colonization in the control group, whereas OA does not affect shNAG-1 cell implanted mice (Fig. 8a–c). Administration of OA rescued the loss of body weight in the control group but failed to do so in shNAG-1 group, suggesting that knock-down of NAG-1 aggravates the progression of HCC metastasis (Fig. 8d). Survival curves demonstrated that mice bearing shNAG-1 cells and the control cells began to die on the 51st and 64th day, respectively. While mice in control group received OA administration began to die on the 77th day, they had a prolonged survival time compared to the other groups (Fig. 8e). Bodyweight analysis and survival curves validated that OA treatment efficiently reduces HCC metastasis in the control group mice but failed in mice with implanted shNAG-1 cells. The expression levels of E-cadherin, Vimentin, and Twist1 in lung tissues were evaluated using immunohistochemistry. In the control group, treatment with OA promoted E-cadherin expression and reduced the expression of Vimentin and Twist1. As compared with the control group, the shNAG-1 group had a lower expression of E-cadherin and significantly higher expression of Vimentin and Twist1. Also, the administration of OA did not affect the expression levels of EMT-related proteins in the shNAG-1 group (Fig. 8f). These results suggested that knocking down NAG-1 enhances the mobility of HCC cells and that NAG-1 plays an indispensable role in OA’s inhibition of HCC metastasis. Using H&E staining, we observed no significant pathological changes of the liver among the mice of different groups (Fig. 8g).
The mice were intravenously inoculated with shCon/shNAG-1 SMMC-7721 cells. a BLI images and b Representative photographs and H&E staining of lung sections. Scale bar = 4 mm. High magnification pictures (×200) of H&E staining. Scale bar = 200 μm. c Quantification of nodules in lungs from indicated mice (n = 6). d Mice body weight change curves of each group. e Kaplan–Meier survival plots show the effect of OA on the survival of mice (n = 6). Data are represented as mean ± SD. *P < 0.05 and **P < 0.01. Differences are tested using Student’s t-test. f Representative immunohistochemical analysis of NAG-1, E-cadherin, Vimentin, and Twist1 protein levels (brown) in each experimental group. Scale bar: 100 μm and 50 μm. g Representative H&E staining of liver sections. Scale bar = 200 μm
The in vitro results showed that OA regulates the expression of NAG-1 by directly interacting with HDAC1. To validate these results in vivo, we constructed HDAC1 overexpression plasmids and stably transfected SMMC-7721 cells by lentivirus. The efficiency of HDAC1 overexpression in SMMC-7721 cells was then evaluated using Western blot (Supplementary Fig. 5a). We observed increased lung mass colonization in mice bearing pLenti-HDAC1 cells compared with pLenti-Con group mice, while treatment with OA had a limited effect on the pLenti-HDAC1 group mice (Supplementary Fig. 5b, c). So, the overexpression of HDAC1 promoted the metastasis of SMMC-7721 cells and blocked OA-induced anti-metastasis in vivo. These observations suggest that a reduction in HDAC1 expression mediates the anti-metastasis effect of OA.
Discussion
HCC is one of the most lethal tumors in the world. At advanced stages, HCC carries a high risk of metastasis and results in shorter overall survival. OA, a flavonoid derivative with potent bioactivity, has exhibited significant potency in pre-clinical studies [15]. In this study, we report that OA inhibits HCC metastasis in vitro and in vivo and that the expression of NAG-1 plays a crucial role in the process. Further, OA elevated the acetylation level of C/EBPβ by interacting with HDAC1, thereby promoting the expression of NAG-1 (Fig. 7g).
EMT is a developmental phenomenon that enables the carcinoma cells to acquire attributes of invasion and metastasis [26,27,28,29]. TGF-β1 acts as a promoter at advanced stages of tumor progression, paving the way for the initiation of EMT. Sources of TGF-β1 in tumors vary and include the cancer cells themselves as well as tumor stroma [8]. In our study, OA inhibited the metastasis of HCC cells in the presence or absence of exogenous TGF-β1. Although exogenous TGF-β1 was absent, we hypothesized that a certain amount of TGF-β1 derived from the serum of culture medium and autocrine of HCC cells must have been present in our culture system. Phosphorylation of Smad is known to propagate TGF-β signal in cells. We found that OA treatment did not affect TGF-β1-induced phosphorylation of Smad2/3 and its nuclear translocation, but the binding of Smad complex to DNA was reduced. NAG-1 inhibits TGF-β1 signaling by interrupting the DNA binding activity of the Smad complex [9]. We observed that OA induced NAG-1 expression in the cytoplasm and nucleus in a dose-dependent manner. The downregulation of NAG-1 increased the DNA binding activity of the Smad complex. Therefore, we hypothesize that NAG-1 will act as a key point in HCC therapy. In this study, NAG-1 induction was found to be implicated in anti-HCC metastasis. Downregulation of NAG-1 promoted the metastatic ability and EMT of HCC cells. At the same time, overexpression of NAG-1 showed the opposite results. We further demonstrate that silencing of NAG-1 abolishes OA-induced repression of metastasis in vivo, suggesting that NAG-1 plays an indispensable role in HCC development.
Multiple mechanisms regulate the basal transcription of NAG-1. Various transcription factors involved in the regulation, such as p53, C/EBPβ, EGR1, and Sp1, have been identified [30]. This study found that C/EBPβ was critical to OA-enhanced transcription of NAG-1 in HCC cell lines. These observations suggest that NAG-1 might be a target gene of C/EBPβ. C/EBPβ play a crucial role in controlling the cell cycle, and promoting metastasis in multiple carcinomas [23]. For instance, C/EBPβ is vital for liver physiology, liver disease, and liver development [31]. Hui et al. found that OA increases the protein expression of C/EBPβ in leukemia cells [32]. However, in HCC cells, we found that OA did not affect expression, phosphorylation, and nuclear translocation of C/EBPβ but increases its transcriptional activity. Further, OA promoted the binding of C/EBPβ to NAG-1’s promoter. Based on these observations, we concluded that OA regulates C/EBPβ at the posttranslational level in HCC cells.
Protein acetylation contributes to some critical protein interaction with DNA and/or other proteins [33]. A disrupted equilibrium with the dominance of the deacetylases leads to the transcriptional repression of genes [34, 35]. Silencing of promoters of various tumor suppressor genes such as p53, C/EBPα, and GSK3β increases the C/EBPβ-HDAC1 complex levels and contributes to the development of HCC [36]. Here, we showed that OA-induced downregulation of HDAC1 in HCC cells increases the acetylation level of C/EBPβ, promoting its transcription activity.
Previous studies show that histone modification plays a critical role in NAG-1 expression in glioblastoma cell lines. Trichostatin A (TSA), a histone deacetylase inhibitor, increases NAG-1 promoter activity and mRNA stability [37]. In our study, OA downregulated the HDAC1 expression. Using molecular docking approaches, we identified several potential active sites for studying the binding between OA and HDAC1. OA demonstrated a moderate affinity with HDAC1 as compared with commercial HDAC inhibitors. Classical HDAC inhibitors such as TSA and suberoylanilide hydroxamic acid (SAHA) inhibit the HDAC activity by interacting with the catalytic sites of HDACs, thereby blocking the substrate access [38, 39]. OA is a natural compound isolated from natural flavonoids with a broad spectrum of pharmacological functions and low toxicity. We found that the proteasome degradation pathway is involved in the OA-reduced downregulation of HDAC1. Using MST and biotin-pulldown assays, we found that Gly149 residue of HDAC1 is essential for its binding with OA. Compared with HDAC1-WT protein, HDAC1-G149A protein is more resistant to degradation by OA because of its weaker interactions with OA. Taken together, we conclude that the Gly149 site is critical for the stability of HDAC1 during its interaction with OA.
Conclusion
In conclusion, OA exhibited a notable anti-metastasis effect in HCC cells in vitro and in vivo. This anti-metastasis effect is due to an OA-induced increase in NAG-1 expression levels. Our study revealed a previously unknown mechanism underlying the tumor-suppressive effect of OA and provided a foundation for further investigation of potential therapeutic strategies to target NAG-1 in HCC.
References
Balogh J, Victor D 3rd, Asham EH, Burroughs SG, Boktour M, Saharia A, et al. Hepatocellular carcinoma: a review. J Hepatocell Carcinoma. 2016;3:41–53.
Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, et al. Hepatocellular carcinoma. Nat Rev Dis Prim. 2016;2:16018.
Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200–7.
Giannelli G, Koudelkova P, Dituri F, Mikulits W. Role of epithelial to mesenchymal transition in hepatocellular carcinoma. J Hepatol. 2016;65:798–808.
Cantelli G, Crosas-Molist E, Georgouli M, Sanz-Moreno V. TGFBeta-induced transcription in cancer. Semin Cancer Biol. 2017;42:60–9.
Massague J. TGFbeta in cancer. Cell. 2008;134:215–30.
Ikushima H, Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nat Rev Cancer. 2010;10:415–24.
Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–72.
Min KW, Liggett JL, Silva G, Wu WW, Wang R, Shen RF, et al. NAG-1/GDF15 accumulates in the nucleus and modulates transcriptional regulation of the Smad pathway. Oncogene. 2016;35:377–88.
Mimeault M, Batra SK. Divergent molecular mechanisms underlying the pleiotropic functions of macrophage inhibitory cytokine-1 in cancer. J Cell Physiol. 2010;224:626–35.
Liu XY, Chi XM, Gong QL, Gao L, Niu YQ, Chi XJ, et al. Association of serum level of growth differentiation factor 15 with liver cirrhosis and hepatocellular carcinoma. PLoS One. 2015;10:e0127518.
Zhang XB, Kang Y, Huo TX, Tao R, Wang XP, Li ZY, et al. GL-V9 induced upregulation and mitochondrial localization of NAG-1 associates with ROS generation and cell death in hepatocellular carcinoma cells. Free Radic Biol Med. 2017;112:49–59.
Yu J, Shen B, Chu ES, Teoh N, Cheung KF, Wu CW, et al. Inhibitory role of peroxisome proliferator-activated receptor gamma in hepatocarcinogenesis in mice and in vitro. Hepatology. 2010;51:2008–19.
Zimmers TA, Gutierrez JC, Koniaris LG. Loss of GDF-15 abolishes sulindac chemoprevention in the ApcMin/+ mouse model of intestinal cancer. J Cancer Res Clin Oncol. 2010;136:571–6.
Lu L, Guo QL, Zhao L. Overview of oroxylin A: a promising flavonoid compound. Phytother Res. 2016;30:1765–74.
Li HB, Chen F. Isolation and purification of baicalein, wogonin and oroxylin A from the medicinal plant Scutellaria baicalensis by high-speed counter-current chromatography. J Chromatogr A. 2005;1074:107–10.
Sun Y, Lu N, Ling Y, Gao Y, Chen Y, Wang L, et al. Oroxylin A suppresses invasion through down-regulating the expression of matrix metalloproteinase-2/9 in MDA-MB-435 human breast cancer cells. Eur J Pharmacol. 2009;603:22–8.
Wei LB, Yao YY, Zhao K, Huang YJ, Zhou YX, Zhao L, et al. Oroxylin A inhibits invasion and migration through suppressing ERK/GSK-3beta signaling in snail-expressing non-small-cell lung cancer cells. Mol Carcinog. 2016;55:2121–34.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Samarakoon R, Overstreet JM, Higgins PJ. TGF-beta signaling in tissue fibrosis: redox controls, target genes and therapeutic opportunities. Cell Signal. 2013;25:264–8.
Wrana JL, Attisano L, Carcamo J, Zentella A, Doody J, Laiho M, et al. TGF beta signals through a heteromeric protein kinase receptor complex. Cell. 1992;71:1003–14.
Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–84.
Zahnow CA. CCAAT/enhancer-binding protein beta: its role in breast cancer and associations with receptor tyrosine kinases. Expert Rev Mol Med. 2009;11:e12.
Cesena TI, Cui TX, Subramanian L, Fulton CT, Iniguez-Lluhi JA, Kwok RP, et al. Acetylation and deacetylation regulate CCAAT/enhancer binding protein beta at K39 in mediating gene transcription. Mol Cell Endocrinol. 2008;289:94–101.
Weerasinghe SV, Estiu G, Wiest O, Pflum MK. Residues in the 11 A channel of histone deacetylase 1 promote catalytic activity: implications for designing isoform-selective histone deacetylase inhibitors. J Med Chem. 2008;51:5542–51.
Klymkowsky MW, Savagner P. Epithelial-mesenchymal transition: a cancer researcher’s conceptual friend and foe. Am J Pathol. 2009;174:1588–93.
Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–73.
Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–42.
Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15–33.
Wang XY, Baek SJ, Eling TE. The diverse roles of nonsteroidal anti-inflammatory drug activated gene (NAG-1/GDF15) in cancer. Biochem Pharmacol. 2013;85:597–606.
Schrem H, Klempnauer J, Borlak J. Liver-enriched transcription factors in liver function and development. Part II: the C/EBPs and D site-binding protein in cell cycle control, carcinogenesis, circadian gene regulation, liver regeneration, apoptosis, and liver-specific gene regulation. Pharmacol Rev. 2004;56:291–330.
Hui H, Chen Y, Yang H, Zhao K, Wang Q, Zhao L, et al. Oroxylin A has therapeutic potential in acute myelogenous leukemia by dual effects targeting PPARgamma and RXRalpha. Int J Cancer 2014;134:1195–206.
Guo L, Li X, Tang QQ. Transcriptional regulation of adipocyte differentiation: a central role for CCAAT/enhancer-binding protein (C/EBP) beta. J Biol Chem. 2015;290:755–61.
Xu WS, Parmigiani RB, Marks PA. Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene. 2007;26:5541–52.
Glozak MA, Seto E. Histone deacetylases and cancer. Oncogene. 2007;26:5420–32.
Wang GL, Salisbury E, Shi XR, Timchenko L, Medrano EE, Timchenko NA. HDAC1 promotes liver proliferation in young mice via interactions with C/EBPbeta. J Biol Chem. 2008;283:26179–87.
Yoshioka H, Kamitani H, Watanabe T, Eling TE. Nonsteroidal anti-inflammatory drug-activated gene (NAG-1/GDF15) expression is increased by the histone deacetylase inhibitor trichostatin A. J Biol Chem. 2008;283:33129–37.
Johnstone RW. Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat Rev Drug Discov. 2002;1:287–99.
Finnin MS, Donigian JR, Cohen A, Richon VM, Rifkind RA, Marks PA, et al. Structures of a histone deacetylase homologue bound to the TSA and SAHA inhibitors. Nature. 1999;401:188–93.
Acknowledgements
We are grateful to Dr. Seung Joon Baek, (University of Tennessee, Knoxville, TN, USA) for kindly providing the plasmids pGL3 NAG-1-1086/+41, pGL3 NAG-1-133/+41 and the mutation plasmids pGL3 m C/EBPβ, pGL3 mRARα, pGL3 mXF1, pGL3 mSp1. And Dr. Zhao-qiu Wu (China Pharmaceutical university, Nanjing, China) for kindly providing pGEX-6P-1 plasmids. And Dr. Chang-ying Guo (China Pharmaceutical university, Nanjing, China) for kindly providing pLuciferase-IRES-mCherry plasmids. This work was supported by the General Program of National Natural Science Foundation of China (81772911, 81973522, 81974425, and 81903648); the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX20-0654); the Natural Science Foundation of Jiangsu Province (BK20170744); the Six Talent Peaks Project in Jiangsu Province (SWYY-095).
Author information
Authors and Affiliations
Contributions
LQ and XBZ supervised the project, designed experiment and wrote the manuscript. TXH, XPW, and YH performed experiments and analyzed data. ZY performed in vivo study, BK performed molecular docking study. QLG provided technical assistance of pharmacology. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Huo, Tx., Wang, Xp., Yu, Z. et al. Oroxylin A inhibits the migration of hepatocellular carcinoma cells by inducing NAG-1 expression. Acta Pharmacol Sin 43, 724–734 (2022). https://doi.org/10.1038/s41401-021-00695-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-021-00695-4
Keywords
This article is cited by
-
Oroxylin A-induced Trained Immunity Promotes LC3-associated Phagocytosis in Macrophage in Protecting Mice Against Sepsis
Inflammation (2024)
-
Modulation of hypoxia-inducible factor-1 signaling pathways in cancer angiogenesis, invasion, and metastasis by natural compounds: a comprehensive and critical review
Cancer and Metastasis Reviews (2024)
-
Anticancer potential of oroxylin A: from mechanistic insight to synergistic perspectives
Naunyn-Schmiedeberg's Archives of Pharmacology (2023)