Abstract
Baicalein is a natural flavonoid extracted from the root of Scutellaria baicalensis that exhibits a variety of pharmacological activities. In this study, we investigated the molecular mechanisms underlying the protective effect of baicalein against cardiac hypertrophy in vivo and in vitro. Cardiac hypertrophy was induced in mice by injection of isoproterenol (ISO, 30 mg·kg−1·d−1) for 15 days. The mice received caudal vein injection of baicalein (25 mg/kg) on 3rd, 6th, 9th, 12th, and 15th days. We showed that baicalein administration significantly attenuated ISO-induced cardiac hypertrophy and restored cardiac function. The protective effect of baicalein against cardiac hypertrophy was also observed in neonatal rat cardiomyocytes treated with ISO (10 μM). In cardiomyocytes, ISO treatment markedly increased reactive oxygen species (ROS) and inhibited autophagy, which were greatly alleviated by pretreatment with baicalein (30 μM). We found that baicalein pretreatment increased the expression of catalase and the mitophagy receptor FUN14 domain containing 1 (FUNDC1) to clear ROS and promote autophagy, thus attenuated ISO-induced cardiac hypertrophy. Furthermore, we revealed that baicalein bound to the transcription factor FOXO3a directly, promoting its transcription activity, and transactivated catalase and FUNDC1. In summary, our data provide new evidence for baicalein and FOXO3a in the regulation of ISO-induced cardiac hypertrophy. Baicalein has great potential for the treatment of cardiac hypertrophy.
Similar content being viewed by others
Introduction
Pathological hypertrophy refers to cardiac expansion in response to various unfavorable stimuli, such as myocardial ischemia, hypoxia, diabetes, and hypertension [1, 2]. The underlying mechanisms of pathological hypertrophy include mitochondrial dysfunction, cardiac cell apoptosis, and reactive oxygen species (ROS) production [3]. ROS are metabolites of oxygen species in the body. In the biological state, an appropriate amount of ROS is essential for cell survival. However, in the pathological state, the accumulation of ROS results in mitochondrial dysfunction and energy metabolism, thus promoting a hypertrophic process. Catalase is the main antioxidant agent in cardiac cells under physiological conditions. Maintenance of the dynamic balance between catalase and ROS in cells is crucial for ROS level control and cardiac hypertrophy prevention [4].
The excessive accumulation of ROS destroys cell homeostasis and causes mitochondrial dysfunction, which leads to oxidative stress [5, 6]. In contrast, autophagy can reduce oxidative damage and ROS levels by removing damaged organelles, such as aggregated proteins and mitochondria [7]. Autophagy is a cell-protective process by which damaged cells are degraded in an autophagosome-mediated degradation system [8]. Autophagy is involved in the pathophysiological processes of diverse diseases, such as cancer and muscle diseases [9, 10]. Accumulating evidence has revealed that autophagy plays an important role in regulating cardiac hypertrophy. It has been reported that autophagy attenuates phenylephrine-induced cardiac hypertrophy by inhibiting protein kinase B (Akt)/mammalian target of rapamycin (mTOR) signaling [11, 12]. In contrast, the inhibition of autophagy by lysophosphatidic acid (LPA) exacerbates cardiac hypertrophy during myocardial infarction [13]. In addition, promoting mitochondrial autophagy (also called mitophagy) by knocking out ATPase inhibitory factor 1 (IF1) activates the adenosine 5’-monophosphate (AMP)-activated protein kinase (AMPK) signaling pathway and prevents excessive mitochondrial membrane depolarization, which can inhibit the pathological cardiac hypertrophy induced by isoprenaline in vitro [14]. Similarly, activation of the PTEN-induced putative kinase 1 (PINK1)/Parkin system mediates mitophagy, which eliminates damaged mitochondria and confers compensatory protection to the hypertrophic heart [15]. Therefore, the regulation of autophagy might be a target of treatment for cardiac hypertrophy.
Currently, there is no effective strategy for the treatment of cardiac hypertrophy, and some types of traditional Chinese medicine (TCM) might have cardioprotective effects [16, 17]. However, it is important to clarify the molecular mechanisms of TCM components. Baicalein is a flavonoid extracted from the root of Scutellaria baicalensis. It has a wide range of pharmacological effects, and its functional group is 1,2-catechol [18]. For TCM, baicalein is often identified as a drug that protects the liver and attenuates inflammatory diseases [19]. Moreover, several biological functions of baicalein have been discovered in recent years. For example, baicalein significantly restrains 12-lipoxygenase [20] and exerts antioxidant [21] and anti-inflammatory effects [22], participates in autophagy activation [23, 24], induces cancer cell apoptosis [25] and inhibits cancer cell proliferation and invasion [26, 27]. Since ROS and autophagy play key roles in cardiac hypertrophy, we speculate that the antioxidant properties of baicalein and its capacity to induce autophagy might block the cardiac hypertrophy development by eliminating ROS and damaged mitochondria, which may have the potential to protect the heart from damage and prevent the development of heart failure. Recently, it has been shown that baicalein attenuates cardiac hypertrophy by blocking the mitogen-activated protein kinase (MEK)-extracellular regulated protein kinase (ERK)1/2 signaling pathway [28], as well as the AKT/mTOR, nuclear factor kappa-B (NF-κB) and calcineurin signaling pathways [29]. In addition, baicalein suppresses high mobility group box 1 (HMGB1) release and matrix metalloprotein (MMP)−2/−9 expression in heart tissues with lipopolysaccharide (LPS)-induced hypertrophy [30]. Recent studies demonstrated that baicalein increased the expression of FOXO3a in human non-small cell lung cancer cells [31]. It has been reported that FOXO3a-dependent antioxidant defense mechanisms block various types of stimulus-induced cardiac hypertrophy [32]. While numerous studies have clarified the function of baicalein, its roles in cardiac hypertrophy as mediated by FOXO3a remain unclear, and many mechanisms have yet to be identified.
In this study, we demonstrate that baicalein attenuates ISO-induced cardiac hypertrophy in both cardiomyocytes and mouse heart models. Baicalein inhibited the ROS burst and activated autophagy in the ISO-induced cardiomyocytes. In addition, FOXO3a was found to be a direct target for baicalein through transactivating catalase and FUNDC1, which are antioxidant and autophagy-related genes, respectively. Catalase facilitated the attenuation of excess ROS, and FUNDC1 activated impaired autophagy, which attenuated the development of cardiac hypertrophy. Our results identified a novel cardiac hypertrophy pathway involving baicalein-FOXO3a, which might provide valuable insight into the prevention and treatment of cardiac hypertrophy.
Materials and methods
Materials
Baicalein (purity > 98%, #465119) was purchased from Sigma-Aldrich (St. Louis, MO, USA). BAI was dissolved in dimethyl sulfoxide (DMSO) to generate a 0.1 M stock solution, which was stored at −20 °C. Fetal bovine serum (FBS) was obtained from ExCellBio (Shanghai, China). Anti-catalase (#12980), anti-FOXO3a (#12829) and anti-LC3 (#4108) primary antibodies were purchased from Cell Signaling Technology (Billerica, CA, USA). The anti-FUNDC1 antibody (A16318) and anti-p62 (A11483) were purchased from ABclonal Technology (Wuhan, China). The anti-β-actin antibody (sc-47778) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Ad-mRFP-GFP-LC3 was obtained from Hanbio (Shanghai, China). 3-Methyladenine (3-MA) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Pronase (10196521001) was purchased from Roche (Basel, Switzerland).
Cell culture and treatment
Neonatal rat cardiomyocytes were isolated from 1- to 2-day-old Sprague Dawley rats which were purchased from Daren Fucheng Animal Technology (Qingdao, China), as we described in our previous study [33]. Briefly, the rat hearts were washed and shredded in HEPES-buffered saline solution (pH 7.35) after dissection. The disintegrated tissue was then dispersed in a series of HEPES-buffered saline solution containing 1.2 mg/mL pancreatin and 0.14 mg/mL collagenase type II and incubated at 37 °C (Sigma, St. Louis, MO, USA). After centrifugation, the cells were resuspended in Dulbecco’s modified Eagle’s medium/F-12 (GIBCO, Grand Island, NY, USA) containing 5% heat-inactivated horse serum, 0.1 mM ascorbate, insulin transfer-sodium selenite medium supplement, 100 units/mL penicillin, 100 μg/mL streptomycin and 0.1 mM bromodeoxyuridine. The dissociated cells were preplated for 1 h at 37 °C to obtain cardiomyocytes. The cells were then diluted to 1 × 106 cells/mL and seeded in different culture dishes according to different experimental requirements. For the administration of baicalein, the cells were pretreated for 4 h with baicalein prior to ISO treatment for 24 h.
F-actin staining and cell surface area measurement
After treatment, the cells were fixed with 4% paraformaldehyde in PBS for 15 min followed by permeabilization with 0.05% Triton-X in PBS for 5 min. FITC phalloidin (Yeasen, Shanghai, China) was used to stain F-actin. The cells were observed by confocal microscopy (Leica, Hesse, Germany). The surface area of the F-actin-stained cells was measured using ImageJ software. Thirty cardiomyocytes per field were examined in each experiment.
Adenovirus infection
Adenovirus carrying FOXO3a and adenovirus carrying β-galactosidase (β-gal) were obtained as we described previously [34]. Adenoviral infection of cells was performed as we described previously [35].
Cell transfection with plasmids or siRNAs
The FUNDC1-overexpressing pcDNA3.1 plasmid was purchased from The Beijing Genomics Institute. The FOXO3a-siRNA sequence was 5’-GAGCTCTTGGTGGATCATC-3’. The FOXO3a scramble (FOXO3a-sc) sequence was 5’-CAGUACUUUUGUGUAGUACAA-3’. The FUNDC1-siRNA sequence was 5’-GCAGCACCUGAAAUCAACATT-3’. The FUNDC1 scramble (FUNDC1-sc) sequence was 5’-UUCUCCGAACGUGUCACGUTT-3’. The specificity of the oligonucleotides was confirmed by comparing them with all other sequences in the NCBI database using nucleotide BLAST. The neonatal cardiomyocytes were transfected with Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) using Opti-MEM reduced serum medium (Gibco, Carlsbad, CA, USA).
Quantitative real-time PCR (qRT-PCR)
qRT-PCR was carried out to evaluate the mRNA expression levels of the ANP, BNP, catalase, and FUNDC1 mRNA. Total RNA was isolated using TRIzol (Sigma, Louis, MO, USA). RNA was reverse transcribed using a PrimeScript RT reagent kit (TaKaRa, Otsu, Japan). The primers used are presented in Table 1.
Immunoblotting
Immunoblotting was performed to determine the expression levels of catalase, LC3, FOXO3a, and FUNDC1. Briefly, cells were lysed for 1 h at 4 °C in RIPA buffer (Epizyme, Shanghai, China) containing a protease inhibitor cocktail and PMSF. The total protein quality of the supernatant was quantified using a BCA protein assay kit, and the total protein was resolved by SDS-polyacrylamide gel electrophoresis followed by transfer to PVDF membranes (Millipore, Boston, MA, USA). Membranes were incubated with the indicated primary antibodies at 4 °C overnight. Horseradish peroxidase secondary antibody was incubated with the PVDF membranes for ~1 h at room temperature. The antigen-antibody complexes were visualized using enhanced chemiluminescence.
ChIP analysis
A ChIP assay was performed according to the procedure described with minor modifications [36]. Briefly, cells were fixed for 12 min at room temperature at a final concentration of 1% formaldehyde. The cross-linking reaction was quenched by treatment with 0.125 M glycine for 5 min. The cells were washed twice with PBS and lysed in lysis buffer for 1 h at 4 °C. The lysate was then subjected to ultrasonic disrupted to generate chromatin fragments with an average length from 400 to 800 bp. Then, the sample was prewashed with protein-A/G agarose (Santa Cruz, Santa Cruz, CA, USA) at 4 °C for 1 h with rocking, and then, 5 μg of specific antibody was added, and the cells were rocked overnight at 4 °C. The DNA fragments were purified using a QIAquick Spin Kit (QIAGEN, Hilden, Germany) and were then used as templates for PCR amplification. To analyze the region of FOXO3a-binding sites in the FUNDC1 promoter, PCR was carried out using primers that encompassed the FOXO3a BS1 or BS2 in the FUNDC1 promoter. The primers for BS1 were forward: 5’-AAAATCAAACACAGCAACAGAGTGA-3’; reverse: 5’-TTTTCCTTAGTGCCCTGTTAGTTGA-3’. The primers for BS2 were forward: 5’-GAAGAGCATCCTGAATAAAGACTTG-3’; reverse: 5’-CGTGTTTACTTATGGGAAATGTAGT-3’.
Luciferase activity assay
Luciferase activity assays were performed using a dual-luciferase reporter assay system (Promega, Madison, WI, USA) according to the manufacturer’s instructions. The pGL4.17-FUNDC1 or pGL4.17-FUNDC1-mut construct was transfected into cells using Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA). Then, the cells were infected with adenovirus or transfected with siRNA targeting FOXO3a. Luciferase activity was measured 24 h after infection.
Autophagosome assay
Neonatal cardiomyocytes were transfected with an adenovirus containing mRFP-GFP tandem fluorescently tagged LC3 for 24 h at an MOI of 100. Then, the neonatal cardiomyocytes were exposed to hypertrophic stimuli or drug treatment. The autophagosomes were observed by confocal microscopy.
Drug affinity responsive target stability (DARTS)
After washing once with ice-cold PBS, the cells were treated with ice-cold RIPA lysis buffer containing 0.1 mM PMSF and a protease inhibitor. After they were collected with a spatula, the cells were incubated at 4 °C for 10 min. The cell lysate was centrifuged at 13,000 rpm for 10 min, and the supernatant was diluted with lysis buffer to a final protein concentration of 2 mg/mL. The protein lysate was mixed with 10× TNC buffer (500 mM Tris-HCl, pH 8.0; 500 mM NaCl; and 100 mM CaCl2) (Sigma, St. Louis, MO, USA). The lysate in 1× TNC buffer was divided into 1.5-mL tubes and incubated with baicalein (100 μM) for 1 h at room temperature. After incubation, each sample was divided into 50-μL aliquots (50 μg of protein each) and proteolytically hydrolyzed in different concentrations of pronase (25, 50, 125, 250, and 500 ng) and maintained at room temperature for 10 min. After 10 min, 2 μL of ice-cold 20× protease inhibitor cocktail was added to stop proteolysis, and the sample was immediately placed on ice. The digestion was then terminated by adding a 4× sample loading dye and boiling the sample at 95 °C for 10 min. An aliquot of each sample was then loaded onto an SDS-PAGE gel and finally subjected to Western blotting.
Animal experiments
Mice (C57BL/6, male, 8 weeks old) were purchased from Daren Fucheng Animal Technology (Qingdao, China). We allocated the mice into four groups randomly: the control group (saline), the baicalein control group (saline+BAI), the ISO group (ISO) and the baicalein treatment group (ISO + BAI). Briefly, the ISO and baicalein treatment groups received a peritoneal injection of ISO (30 mg·kg−1·d−1) for 15 days; the baicalein treatment group received caudal vein injections of baicalein (25 mg/kg) on the 3rd, 6th, 9th, 12th, and 15th days of the experiments. The respective control and baicalein control groups were injected with the same amount of saline or baicalein for the corresponding times. Before the mice were sacrificed, echocardiography was performed to assess the degree of cardiac hypertrophy and the therapeutic effects of the baicalein treatment. All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of Qingdao University Medical College.
Statistical analysis
The results are shown as the means ± SD. Significant differences between the control and experimental groups were determined using paired Student’s t tests. We used one-way ANOVA for multiple comparisons. A P value <0.05 was considered statistically significant.
Results
Baicalein attenuates ISO-induced cardiac hypertrophy in vivo and in vitro
ISO induces cardiac hypertrophy and has been used to produce stable cardiac hypertrophy models [37, 38]. First, we tested the effect of baicalein on cardiac hypertrophy in a mouse model. We assessed the heart function of the mice using echocardiography (Fig. 1a). Our results showed a significant decrease in the ejection fraction (EF%) and fractional shortening (FS%) of the mice in the ISO group compared with those in the saline group, but this reduction was completely restored in the baicalein group (Fig. 1b). Furthermore, the ISO-induced increase in the cardiac function index was restored by baicalein treatment (Fig. 1c, d). In addition, we observed a significantly increased heart size in the ISO group compared to that in the saline group, and the histopathological examination of cardiac tissue showed that the tissue sections from the ISO group mice had significant damage, myofibrillar degeneration, and fibrosis. However, baicalein significantly prevented ISO-induced pathological changes, with the mice in the baicalein group exhibiting nearly normal heart size and integral cardiac morphology (Fig. 1e). Moreover, the left ventricular/body weight and heart weight/body weight ratios, which are hypertrophy indicators, were significantly reduced in the baicalein group (Fig. 1f). Compared with the ISO group, the atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) levels in the baicalein group were significantly downregulated (Fig. 1g, h), which also indicated the therapeutic effect of baicalein on cardiac hypertrophy mice.
Mice were intraperitoneally injected with ISO (30 mg·kg−1·d−1) or baicalein (25 mg/kg) for 15 days. a Echocardiography was performed to test heart function. b–d EF ejection fraction, FS fractional shortening of the left ventricular diameter, LVPWd diastole left ventricular posterior wall thickness, LVPWs systolic left ventricular posterior wall thickness, LVIDd diastolic left ventricular internal diameter, LVIDs systolic left ventricular internal diameter. e Whole hearts with H&E staining; scale bar, 20 µm. f Left ventricular/body weight and heart/body weight ratios. g and h ANP and BNP mRNA levels. Error bars represent SD. Data are expressed as the means ± SD, n = 5 mice per group. *P < 0.05.
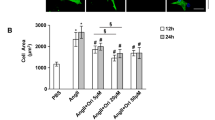
Next, the role of baicalein in ISO-induced cardiac hypertrophy was studied in an in vitro model. The dose of baicalein for cardioprotection is ~20–30 μM [39,40,41]. We verified the dose-dependent effects of baicalein (5–30 μM) on cardiac hypertrophy. We found that 30 μM baicalein significantly inhibited cardiac hypertrophy induction (Supplementary Fig. S3a–c). The cardiomyocytes were treated with 10 μM ISO to induce hypertrophy based on our previous study [38]. After treating the cells with baicalein, we assessed the cell surface area and hypertrophic markers, including ANP and BNP levels. Our results showed that the increases in cell surface area (Fig. 2a, b) and ANP and BNP levels (Fig. 2c, d) induced by ISO were significantly decreased with baicalein treatment. Taken together, these results indicated that baicalein attenuated ISO-induced cardiac hypertrophy.
The neonatal rat cardiomyocytes were treated with 10 μM ISO or 30 μM baicalein for 24 h. Cells were pretreated for 4 h with baicalein prior to ISO treatment. a The cell surface area of neonatal rat cardiomyocytes was assessed by TRITC-conjugated phalloidin staining. Representative photos taken by confocal microscopy; blue, DAPI-stained nuclei; red, phalloidin-stained F-actin; scale bar, 25 µm. b Photos taken with a fluorescence microscope for the statistical analysis of cell surface area. c and d ANP and BNP mRNA levels were detected by real-time PCR. Error bars represent SD. Data are expressed as the means ± SD, n = 3. *P < 0.05.
Baicalein inhibited the ROS burst by promoting catalase in hypertrophic cardiomyocytes induced by ISO
We next attempted to elucidate the mechanism by which baicalein inhibits cardiac hypertrophy. ROS production is increased during cardiac hypertrophy. Thus, we examined the level of ROS in the cardiomyocytes treated with ISO. Our results showed that the levels of ROS increased in a time-dependent manner, indicating ROS production in cardiomyocytes (Fig. 3a). Simultaneously, the protein and mRNA expression levels of catalase were significantly decreased (Fig. 3b, c). Next, we explored whether baicalein was involved in the ISO-induced regulation of catalase in cardiomyocytes. Baicalein significantly attenuated ROS production during hypertrophy induction (Fig. 3d). Interestingly, baicalein also significantly restored the expression levels of catalase (Fig. 3e, f). Therefore, the inhibitory effect of baicalein on the ROS burst during the hypertrophy induced by ISO was achieved by increasing catalase levels.
a–c Neonatal rat cardiomyocytes treated with 10 µM ISO at the indicated times. a ROS levels were detected by DCF-DA staining. The intensity of the DCF-DA fluorescence was measured by a fluorescent enzyme-labeled detection instrument. b Catalase protein levels were determined by Western blotting. c Catalase mRNA levels were determined by real-time PCR. d–f Neonatal rat cardiomyocytes were treated with 10 µM ISO or 30 µM baicalein for 24 h. d Baicalein prevents ISO-induced ROS production. e Baicalein increased the catalase protein levels and f mRNA levels, which were inhibited by ISO. Error bars represent SD. Data are expressed as the means ± SD, n = 3. *P < 0.05.
Baicalein activated autophagy by promoting FUNDC1 in the ISO-induced hypertrophic cardiomyocytes
Autophagy plays a crucial role in regulating cardiac hypertrophy [42,43,44]. Therefore, we tested the autophagy-associated protein levels of LC3 and p62. The LC3II/I levels were significantly decreased in a time-dependent manner (Fig. 4a), and the p62 levels were increased (Supplementary Fig. S5a). The results showed that the formation of autophagosomes and the degradation of autophagosomes were inhibited during the process of cardiac hypertrophy induction. Moreover, we examined the protein expression levels of LC3 after the cardiomyocytes were treated with baicalein. Baicalein significantly promoted the conversion of LC3 I to LC3 II (Fig. 4b) and downregulated the p62 levels accordingly (Supplementary Fig. S4b). We also tested the expression of LC3 and p62 in tissue samples, and the results were consistent with those obtained at the cellular level (Supplementary Fig. S1a, b). To confirm the effect of baicalein on autophagy during cardiac hypertrophy induction, we used an adenovirus expressing mRFP-GFP tandem fluorescently tagged LC3 to detect autophagosome levels. Our results showed that the number of autophagosomes in ISO-induced hypertrophic cardiomyocytes was reduced compared to that of the negative controls. Moreover, the number of autophagosomes was increased after treatment with baicalein during the ISO induction (Fig. 4c, d). To confirm whether baicalein attenuates cardiac hypertrophy by promoting autophagy, we used 3-MA, an inhibitor of autophagy. Our findings indicated that baicalein inhibited the ISO-induced increase in cell surface area and the upregulation of ANP and BNP, whereas this inhibition was abolished by treatment with 3-MA (Supplementary Fig. S4a–c). Our results showed that baicalein activated autophagy during ISO-induced cardiac hypertrophy. Next, we explored the mechanisms by which baicalein promoted autophagy. FUNDC1 is a novel mitophagy receptor that can promote mitophagy and plays a protective role in cardiomyocytes [45, 46]. We observed that FUNDC1 expression decreased in a time-dependent manner during the induction of cardiac hypertrophy (Fig. 4e). We detected an increase in the protein and mRNA expression levels of FUNDC1 after treatment with baicalein in vitro (Fig. 4f, g) and in vivo (Supplementary Fig. S2a, b). To confirm the role of FUNDC1 in cardiac hypertrophy, which was attenuated by baicalein, we knocked down endogenous FUNDC1 using siRNA (Supplementary Fig. S5d). The effect of baicalein on the attenuation of cardiac hypertrophy was abolished by knocking down FUNDC1 (Fig. 4h–j). Taken together, these results indicated that baicalein attenuates cardiac hypertrophy by activating autophagy via FUNDC1.
a LC3 protein levels in neonatal rat cardiomyocytes treated with 10 μM ISO at the indicated times were detected by Western blotting. b LC3 protein levels treated with baicalein. c After treatment with ISO or baicalein for 24 h, neonatal rat cardiomyocytes were transfected with mRFP-GFP-LC3 adenovirus. Representative photos of fluorescent LC3 were taken by confocal microscopy; scale bar, 25 µm. d The numbers of GFP and RFP puncta in neonatal rat cardiomyocytes; green puncta, autophagosomes; red puncta, autolysosomes. Autophagosomes fuse with autolysosomes to complete the autophagy process. The remaining red puncta represent the degree of autophagy, which was detected by the difference in area, excluding the overlapping areas of red and green puncta (yellow puncta). e FUNDC1 protein levels in the neonatal rat cardiomyocytes treated with 10 µM ISO at the indicated times. f Baicalein increased the FUNDC1 protein levels and g mRNA levels, which were inhibited by ISO. h Baicalein inhibited the increases in cell surface area and (i and j) ANP and BNP mRNA expression levels, which were abolished by the simultaneous knockdown of FUNDC1. Error bars represent SD. Data are expressed as the means ± SD, n = 3. *P < 0.05.
FUNDC1 was involved in the regulation of ISO-induced cardiac hypertrophy
We confirmed that baicalein promotes the expression of FUNDC1 in ISO-induced cardiac hypertrophy and that FUNDC1 is a key regulator of baicalein to attenuate cardiac hypertrophy. To confirm the role of FUNDC1 in cardiac hypertrophy, we overexpressed FUNDC1 in cardiomyocytes to confirm its role in the induction of cardiac hypertrophy (Fig. 5a). FUNDC1 overexpression promoted the conversion of LC3 I to LC3 II (Fig. 5b) and downregulated the p62 levels accordingly (Fig. 5c). Moreover, it inhibited ISO-induced cardiac hypertrophy by decreasing the cell surface area (Fig. 5d) and by downregulating the levels of ANP and BNP (Fig. 5e, f). These results confirmed that FUNDC1-mediated autophagy was involved in the protection of ISO-induced cardiac hypertrophy.
a A FUNDC1 overexpression vector efficiently forced the expression of FUNDC1 in neonatal rat cardiomyocytes. b FUNDC1 overexpression promoted the conversion of LC3 I to LC3 II and c downregulated p62 levels in neonatal rat cardiomyocytes exposed to 10 μM ISO. d FUNDC1 overexpression inhibited the increase in cell surface area in the neonatal rat cardiomyocytes exposed to 10 μM ISO for 24 h. e and f FUNDC1 overexpression inhibited the increase in ANP and BNP mRNA expression levels. Error bars represent SD. Data are expressed as the means ± SD, n = 3. *P < 0.05.
Baicalein exerted its cardioprotective effects by binding directly to FOXO3a
In ISO-induced cardiac hypertrophy contexts, we observed that the protein and mRNA expression levels of catalase and FUNDC1 were upregulated by baicalein. Thus, we hypothesized that baicalein activated the transcription of genes involved in hypertrophy through transcription factors. According to recent reports and our previous studies, we found that the transcriptional activity of the transcription factor FOXO3a plays an important role in cardiac hypertrophy [47, 48]. First, we examined the expression of FOXO3a in ISO-induced cardiac hypertrophy and found that FOXO3a expression was significantly decreased in a time-dependent manner (Fig. 6a). Then, we analyzed the protein expression levels of FOXO3a after treatment with baicalein in vitro (Fig. 6b) and in vivo (Supplementary Fig. S2c). The results showed that baicalein significantly promoted FOXO3a expression. To determine whether baicalein regulates FOXO3a, we simulated the binding of baicalein to FOXO3a using molecular docking. Our analysis showed that baicalein and FOXO3a established hydrogen bonds at the GLU171, ILE236 and LYS176 sites and pi bonds at the GLU171 and ILE236 sites (Fig. 6c). To verify the interaction of baicalein and FOXO3a, we used a drug affinity responsive target stability (DARTS) assay to obtain more evidence. Pronase is a nonspecific proteolytic enzyme that can hydrolyze all the proteins in the sample, including FOXO3a. It was used to test whether baicalein enables FOXO3a resistance to hydrolysis. Our results showed that FOXO3a protein levels were downregulated upon treatment with pronase (Fig. 6d). However, the baicalein treatment obviously attenuated the decrease in FOXO3a protein levels (Fig. 6e). These findings indicated that baicalein has the capacity to bind to FOXO3a. To further confirm the regulation of FOXO3a by baicalein, we used siRNA to knock down FOXO3a in the cardiomyocytes (Fig. 6f). Our results showed that baicalein inhibited the ISO-induced increase in myocardial cell surface area (Fig. 6g) and the upregulation of ANP and BNP levels (Fig. 6h, i); this inhibition was abolished by the simultaneous knockdown of FOXO3a. Taken together, these results demonstrated that baicalein can attenuate ISO-induced cardiac hypertrophy by targeting FOXO3a.
a The FOXO3a protein levels in neonatal rat cardiomyocytes treated with 10 μM ISO at the indicated times were detected by Western blotting. b FOXO3a protein levels in neonatal rat cardiomyocytes treated with 10 μM ISO or 30 μM baicalein were detected by Western blotting. c Molecular docking predicted baicalein targeting of FOXO3a. d Level of FOXO3a protein in neonatal rat cardiomyocytes treated with different concentrations of pronase as indicated. e Level of FOXO3a protein in the neonatal rat cardiomyocytes cotreated with pronase and baicalein. f siRNA transfection efficiently knocked down endogenous FOXO3a expression. Baicalein inhibited the increases in (g) cell surface area and (h and i) ANP and BNP mRNA expression levels, which were abolished by the simultaneous knockdown of FOXO3a. Error bars represent SD. Data are expressed as the means ± SD, n = 3. *P < 0.05.
FOXO3a transcriptionally regulated catalase to inhibit ROS in the ISO-induced hypertrophic cardiomyocytes
Since FOXO3a is a downstream regulator of baicalein, we explored the role of FOXO3a in oxidative stress and autophagy. We overexpressed FOXO3a in the cardiomyocytes (Fig. 7a). Our results showed that FOXO3a overexpression in the cardiomyocytes significantly inhibited ISO-induced ROS production (Fig. 7b). Moreover, the protein and mRNA expression levels of catalase were upregulated accordingly (Fig. 7c, d). Taken together, these results demonstrated that FOXO3a can inhibit ROS bursts by promoting catalase expression at the transcriptional level during the induction of cardiac hypertrophy. In addition, FOXO3a overexpression activated autophagy (Fig. 7e, Supplementary Fig. S5c). Taken together, our results show that FOXO3a, which is a downstream target of baicalein, can promote catalase activity to inhibit ROS bursts and can activate autophagy when ISO is used to induce cardiac hypertrophy.
a Adenovirus (MOI = 50 or 100) efficiently forced the expression of FOXO3a in neonatal rat cardiomyocytes. b Forcing the expression of FOXO3a prevented an ISO-induced ROS burst. c Forcing the expression of FOXO3a restored the protein and d mRNA expression levels of catalase. e Forcing the expression of FOXO3a promoted the conversion of LC3 I to LC3 II. Error bars represent SD. Data are expressed as the means ± SD, n = 3. *P < 0.05.
FOXO3a targeted FUNDC1 to regulate autophagy in the ISO-induced hypertrophic cells
We examine whether FOXO3a can directly transactivate FUNDC1 to inhibit cardiac hypertrophy. To this end, we found that the promoter region of FUNDC1 has two potential binding sites for FOXO3a (Fig. 8a). A chromatin immunoprecipitation (ChIP) analysis showed that FOXO3a binds to the binding site 2 (BS2) region, which is inhibited by ISO, but does not interact with the binding site 1 (BS1) region (Fig. 8b). To further test whether FOXO3a can regulate FUNDC1 promoter activity, we constructed a luciferase vector containing two potential binding sites, Wt1, and a vector containing the BS2-only fragment, Wt2. Our results showed that the overexpression of FOXO3a induced luciferase activity in the cells transfected with the Wt1 and Wt2 promoters. Compared with the change to BS1, BS2 showed no change in luciferase activity, indicating that BS1 is not a binding site. Luciferase activity was significantly lost after the introduction of a BS2 mutation (m-BS2) (Fig. 8c). Therefore, the binding site of FOXO3a is in the BS2 region of the FUNDC1 promoter. Then, we used siRNA to knock down the expression of endogenous FOXO3a. Our results showed that knocking down FOXO3a led to a decrease in luciferase activity (Fig. 8d). Simultaneously, we examined the effect of ISO on the activity of the FUNDC1 promoter. The results showed that the ISO treatment significantly inhibited luciferase activity (Fig. 8e). Taken together, these results indicated that FOXO3a can transactivate FUNDC1.
a The promoter region of FUNDC1 contains two potential FOXO3a-binding sites (BS1 and BS2). The FUNDC1 promoter fragments Wt1 (containing BS1 and BS2) and Wt2 (BS2 only) were linked to a luciferase reporter gene. Mutations were introduced into BS2 in Wt1 (m-BS2). b ChIP analysis of FOXO3a binding to the promoter of FUNDC1. Neonatal rat cardiomyocytes were treated with 10 µM ISO for 24 h. c Luciferase activity detected in the HEK-293 cells infected with FOXO3a adenovirus or β-gal with the luciferase reporter constructs as indicated. d Knockdown of endogenous FOXO3a reduced FUNDC1 promoter luciferase activity upon siRNA transfection. e ISO treatment resulted in reduced luciferase activity of the FUNDC1 promoter. Forcing the expression of FOXO3a increased the (f) protein and (g) mRNA expression levels of FUNDC1, which had been inhibited by ISO. h Overexpression of FOXO3a promoted the conversion of LC3 I to LC3 II, which was abolished by the simultaneous knockdown of FUNDC1 in the neonatal rat cardiomyocytes treated with 10 µM ISO for 24 h. Overexpression of FOXO3a inhibited the (i) increases in cell surface area and (j and k) ANP and BNP mRNA expression levels, which were abolished by simultaneous knockdown of FUNDC1. Error bars represent SD. Data are expressed as the means ± SD, n = 3. *P < 0.05.
Since FUNDC1 is a transcription target of FOXO3a, it can activate autophagy during ISO-induced cardiac hypertrophy. The overexpression of FOXO3a significantly upregulated the mRNA and protein expression levels of FUNDC1 in ISO-induced cardiac hypertrophy (Fig. 8f, g). Therefore, we hypothesized that the role of FOXO3a in inhibiting cardiac hypertrophy by activating autophagy was achieved by the regulation of FUNDC1. To validate our hypothesis, we used siRNA to knock down FUNDC1 in the cardiomyocytes (Supplementary Fig. S5d). Our results showed that the overexpression of FOXO3a promoted ISO-induced autophagy inhibition and that this promotion was abolished by the simultaneous knockdown of FUNDC1 (Fig. 8h, Supplementary Fig. S5e). Furthermore, the overexpression of FOXO3a inhibited the ISO-induced increase in myocardial cell surface area (Fig. 8i) and the upregulation of ANP and BNP levels (Fig. 8j, k), but these effects were simultaneously offset by the knockdown of FUNDC1. Taken together, these results demonstrated that FUNDC1 is a key downstream regulator of FOXO3a in the inhibition of ISO-induced cardiac hypertrophy.
Discussion
Cardiac hypertrophy is a risk factor for many heart diseases, and sustained cardiac hypertrophy causes systolic dysfunction, eventually leading to heart failure, arrhythmia and sudden death [49]. Understanding the potential molecular mechanisms of cardiac hypertrophy will benefit the development of new drugs to prevent and treat heart failure. In the present study, using cardiomyocytes and an isoproterenol-induced cardiac hypertrophy mouse model, we revealed that baicalein treatment in vitro and in vivo attenuates isoproterenol-induced cardiac hypertrophy. We found that baicalein regulates FOXO3a to inhibit ROS and activate autophagy in cardiac hypertrophy. Furthermore, we also confirmed that FOXO3a is the upstream regulator of catalase and FUNDC1 in isoproterenol-induced cardiac hypertrophy and inhibited cardiac hypertrophy by the transactivation of catalase and FUNDC1.
Multiple studies have confirmed that baicalein fulfills an essential function in protecting cardiac cells [40, 50]. It has been reported that baicalein inhibits cardiomyocyte apoptosis induced by doxorubicin and myocardial hypoxia [39, 41]. In addition, baicalein protects cardiomyocytes from mitochondrial oxidative damage associated with Akt activation [51]. Recent studies have shown that baicalein attenuates cardiac hypertrophy. Baicalein was first discovered to attenuate aortic banding (AB)-induced cardiac hypertrophy by blocking MEK-ERK1/2 signaling [28]. Subsequently, it was reported that baicalein attenuated LPS-induced cardiac hypertrophy by inhibiting the HMGB1 and MMP-2/-9 signaling pathways [30]. However, the specific molecular mechanism by which baicalein attenuates cardiac hypertrophy remains largely unknown. Furthermore, baicalein regulates FOXO3a in non-small cell lung cancer cells [31]. Our study confirms that baicalein attenuates cardiac hypertrophy via the FOXO3a-catalase/FUNDC1 signaling pathway. No current evidence previously indicated that baicalein directly targets FOXO3a. We verified that baicalein targets FOXO3a by using molecular docking and a DARTS assay. We propose to conduct more specific research in the future to explore other direct targets of baicalein based on its superior myocardial protection. Baicalein has many targets in the body. For example, baicalein counteracts oxidative stress in cardiomyocytes by disrupting the interaction of nuclear factor erythroid-2-related factor 2 (Nrf2)/Kelch-like ECH-associated protein l (Keap1) [39]. Moreover, baicalein prevents tumor progression by upregulating the expression of DNA damage-inducible transcript 4 (DDIT4) and by targeting arachidonic acid and GTPase [23, 26, 52]. Therefore, the discovery of other targets of baicalein is worthy of pursuit.
FOXO3a is a member of the subfamily forkhead box class O (FOXO), also known as FOXO3 or forehead in rhabdomyosarcoma-like 1 (FKHRL1), and is highly conserved in mammals. FOXO3a is involved in the transcriptional regulation of proteins critical for many different functions in cells [53]. The nuclear localization of FOXO3a can transactivate the transcription of target genes important for cellular biological functions, such as cell cycle-related genes [54], apoptosis genes [55], and antioxidative-related genes [56]. There is increasing evidence suggesting that FOXO3a is critically important to cardiac hypertrophy [32, 57]. It has been demonstrated that FOXO3a transactivates catalase in response to oxidative stress, which inhibits cardiac hypertrophy [48]. In addition, the deacetylation of FOXO3a increases the level of SOD2, which can inhibit ROS expression, blocking ANG II-induced cardiac hypertrophy [57]. Thus, FOXO3a inhibited the hypertrophy stimulation-induced ROS burst, which was achieved by targeting multiple antioxidant genes. However, whether baicalein plays a role in cardiac hypertrophy through FOXO3a to activate antioxidant genes remains unclear. Our work confirmed that isoproterenol can stimulate ROS elevation during hypertrophy induction and that overexpression of FOXO3a attenuates cardiac hypertrophy by upregulating catalase levels through its transcriptional activity. These findings suggest that baicalein activates catalase to eliminate ROS by promoting FOXO3a expression in ISO-induced cardiac hypertrophy.
Interestingly, FOXO3a can also activate autophagy in cardiomyocytes. It has been reported that the poly(ADP-ribosyl)ation of FOXO3a mediates the dissociation of histone H1 from the FOXO3a target gene and promotes the nuclear localization of FOXO3a to activate autophagy-related gene expression, which inhibits cardiomyocyte apoptosis and fibrosis [58]. Moreover, it was reported that FOXO3a-dependent autophagy protected against cardiac hypertrophy induced by pressure overload [59]. Activated autophagy plays an essential role in attenuating the effect of baicalein on cardiac hypertrophy. Our data showed that baicalein targeted FOXO3a. As expected, the combination of baicalein and FOXO3a increased FOXO3a stability and prevented its degradation, which contributed to FOXO3a transactivation of autophagy-related genes. Furthermore, it was reported that the transcriptional activity of FOXO3a activated the transcription of its downstream target protein SOD2, which attenuated ANG II-induced cardiac hypertrophy by inhibiting ROS levels and enhancing mitochondrial function [60, 61]. These findings suggest a potential relationship between ROS and mitochondrial autophagy. The burst of ROS causes mitochondrial damage, leading to abnormal energy metabolism, and the initiation of mitochondrial autophagy clears damaged mitochondria, thereby attenuating cardiac hypertrophy. FOXO3a may also upregulate the expression of its downstream ubiquitin ligase muscle atrophy F-box (MAFbx), which hydrolyzes excess protein in cardiomyocytes to attenuate cardiac hypertrophy [62]. Similarly, FOXO3a mediates cardiomyocyte resistance to hypertrophy by initiating the transcription of its downstream target p21 [63]. In summary, FOXO3a-dependent autophagy might be an important target for the treatment of cardiac hypertrophy. Our study confirmed that baicalein can target FOXO3a, which is a critical transcription factor involved in the activation of antioxidant and autophagy genes modulating cardiac hypertrophy; therefore, baicalein is of great significance in the treatment of cardiac hypertrophy.
FUNDC1 is a newly identified mitochondrial autophagy receptor that recruits LC3 to mitochondria to initiate mitochondrial autophagy [64, 65]. Mitochondria are the critical organelles of cellular energy metabolism, and mitochondrial dysfunction has been shown to be associated with pathological hypertrophy [66]. As a mitochondrial autophagy regulatory protein, FUNDC1 was recently reported to be closely associated with cardiac disease. In a mouse model of ischemia/reperfusion, FUNDC1-mediated mitochondrial autophagy regulated the number and quality of mitochondria by degrading damaged mitochondria, protecting cardiomyocytes from I/R injury [45]. Various types of hypertrophy stimulation-induced oxidative stress are major factors of mitochondrial damage, which disrupts mitochondrial function and affects energy metabolism, further exacerbating cardiac hypertrophy [67]. Recent reports have shown that mammalian STE20-like kinase 1 (Mst1) upregulates ROS in cardiomyocytes, thereby downregulating FUNDC1 expression and inhibiting mitochondrial autophagy. In contrast, Mst1 knockdown reverses FUNDC1-mediated mitochondrial autophagy, thus reducing cardiomyocyte apoptosis upon I/R injury [68]. Surprisingly, we found that FOXO3a activates FUNDC1 through its transcriptional activity to promote autophagy in ISO-induced cardiac hypertrophy. However, the role of mitochondrial division and fusion in cardiac hypertrophy, and the determination of whether FOXO3a-mediated FUNDC1-dependent autophagy is truly mitochondrial autophagy requires further exploration. Excessive autophagy can aggravate cardiac hypertrophy. It has been reported that excessive autophagy induced by external stimuli, such as transverse aortic constriction (TAC), phenylephrine and ANG II, promotes the progression of cardiac hypertrophy [69,70,71]. Our studies indicated that autophagy was inhibited upon ISO-induced cardiac hypertrophy. Therefore, we believe that FOXO3a restores impaired autophagy by activating FUNDC1, leading to a beneficial effect that attenuates ISO-induced cardiac hypertrophy. This finding suggests that FUNDC1 might become a new therapeutic target for heart disease, especially cardiac hypertrophy. In the future, further investigations need to be implemented to explore the molecular mechanisms.
In conclusion, our data revealed the therapeutic role of baicalein in ISO-induced cardiac hypertrophy. Baicalein attenuates ISO-induced cardiac hypertrophy by promoting the transcriptional activity of FOXO3a. FOXO3a transactivates the antioxidation gene catalase to inhibit ROS bursts, while the autophagy-associated gene FUNDC1 is also transactivated to activate impaired autophagy, which might provide a potential therapeutic strategy for cardiac hypertrophy.
References
Bernardo BC, Weeks KL, Pretorius L, McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther. 2010;128:191–227.
Li Z, Wang J, Yang X. Functions of autophagy in pathological cardiac hypertrophy. Int J Biol Sci. 2015;11:672–8.
Nakamura M, Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol. 2018;15:387–407.
Xu FP, Chen MS, Wang YZ, Yi Q, Lin SB, Chen AF, et al. Leptin induces hypertrophy via endothelin-1-reactive oxygen species pathway in cultured neonatal rat cardiomyocytes. Circulation 2004;110:1269–75.
Chakrabarti S, Jahandideh F, Wu J. Food-derived bioactive peptides on inflammation and oxidative stress. Biomed Res Int. 2014;2014:608979.
Fandy TE, Jiemjit A, Thakar M, Rhoden P, Suarez L, Gore SD. Decitabine induces delayed reactive oxygen species (ROS) accumulation in leukemia cells and induces the expression of ROS generating enzymes. Clin Cancer Res. 2014;20:1249–58.
Ureshino RP, Rocha KK, Lopes GS, Bincoletto C, Smaili SS. Calcium signaling alterations, oxidative stress, and autophagy in aging. Antioxid Redox Signal. 2014;21:123–37.
Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–73.
Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–77.
Cuervo AM. Autophagy: in sickness and in health. Trends Cell Biol. 2004;14:70–77.
Liu C, Xue R, Wu D, Wu L, Chen C, Tan W, et al. REDD1 attenuates cardiac hypertrophy via enhancing autophagy. Biochem Biophys Res Commun. 2014;454:215–20.
Xue R, Zeng J, Chen Y, Chen C, Tan W, Zhao J, et al. Sestrin 1 ameliorates cardiac hypertrophy via autophagy activation. J Cell Mol Med. 2017;21:1193–205.
Yang J, Xu J, Han X, Wang H, Zhang Y, Dong J, et al. Lysophosphatidic acid is associated with cardiac dysfunction and hypertrophy by suppressing autophagy via the LPA3/AKT/mTOR pathway. Front Physiol. 2018;9:1315.
Yang K, Long Q. Knockout of the ATPase inhibitory factor 1 protects the heart from pressure overload-induced cardiac hypertrophy. Sci Rep. 2017;7:10501.
Xiong W, Hua J, Liu Z, Cai W, Bai Y, Zhan Q, et al. PTEN induced putative kinase 1 (PINK1) alleviates angiotensin II-induced cardiac injury by ameliorating mitochondrial dysfunction. Int J Cardiol. 2018;266:198–205.
D’Amico R, Fusco R, Gugliandolo E, Cordaro M, Siracusa R, Impellizzeri D, et al. Effects of a new compound containing palmitoylethanolamide and baicalein in myocardial ischaemia/reperfusion injury in vivo. Phytomedicine. 2019;54:27–42.
Liu R, Zhang HB, Yang J, Wang JR, Liu JX, Li CL. Curcumin alleviates isoproterenol-induced cardiac hypertrophy and fibrosis through inhibition of autophagy and activation of mTOR. Eur Rev Med Pharmacol Sci. 2018;22:7500–8.
Tsou LK, LaraTejero M, RoseFigura J, Zhang ZJ, Wang YC, Yount JS, et al. Antibacterial flavonoids from medicinal plants covalently inactivate type III protein secretion substrates. J Am Chem Soc. 2016;138:2209–18.
Chen H, Gao Y, Wu J, Chen Y, Chen B, Hu J, et al. Exploring therapeutic potentials of baicalin and its aglycone baicalein for hematological malignancies. Cancer Lett. 2014;354:5–11.
Li Y, Chen Q, Ran D, Wang H, Du W, Luo Y, et al. Changes in the levels of 12/15-lipoxygenase, apoptosis-related proteins and inflammatory factors in the cortex of diabetic rats and the neuroprotection of baicalein. Free Radic Biol Med. 2019;134:239–47.
Shi L, Hao Z, Zhang S, Wei M, Lu B, Wang Z, et al. Baicalein and baicalin alleviate acetaminophen-induced liver injury by activating Nrf2 antioxidative pathway: The involvement of ERK1/2 and PKC. Biochem Pharmacol. 2018;150:9–23.
Dai C, Tang S, Wang Y, Velkov T, Xiao X. Baicalein acts as a nephroprotectant that ameliorates colistin-induced nephrotoxicity by activating the antioxidant defence mechanism of the kidneys and down-regulating the inflammatory response. J Antimicrob Chemother. 2017;72:2562–9.
Wu R, Murali R, Kabe Y, French SW, Chiang YM, Liu S, et al. Baicalein targets GTPase-mediated autophagy to eliminate liver tumor-initiating stem cell-like cells resistant to mTORC1 inhibition. Hepatology. 2018;68:1726–40.
Wang YF, Xu YL, Tang ZH, Li T, Zhang LL, Chen X, et al. Baicalein induces beclin 1- and extracellular signal-regulated kinase-dependent autophagy in ovarian cancer cells. Am J Chin Med. 2017;45:123–36.
Liu W, Wang X, Liu Z, Wang Y, Yin B, Yu P, et al. SGK1 inhibition induces autophagy-dependent apoptosis via the mTOR-Foxo3a pathway. Br J Cancer. 2017;117:1139–53.
Wang Y, Han E, Xing Q, Yan J, Arrington A, Wang C, et al. Baicalein upregulates DDIT4 expression which mediates mTOR inhibition and growth inhibition in cancer cells. Cancer Lett. 2015;358:170–9.
Wang L, Ling Y, Chen Y, Li CL, Feng F, You QD, et al. Flavonoid baicalein suppresses adhesion, migration and invasion of MDA-MB-231 human breast cancer cells. Cancer Lett. 2010;297:42–48.
Zong J, Zhang DP, Zhou H, Bian ZY, Deng W, Dai J, et al. Baicalein protects against cardiac hypertrophy through blocking MEK-ERK1/2 signaling. J Cell Biochem. 2013;114:1058–65.
Wang AW, Song L, Miao J, Wang HX, Tian C, Jiang X, et al. Baicalein attenuates angiotensin II-induced cardiac remodeling via inhibition of AKT/mTOR, ERK1/2, NF-kappaB, and calcineurin signaling pathways in mice. Am J Hypertens. 2015;28:518–26.
Chen HM, Liou SF, Hsu JH, Chen TJ, Cheng TL, Chiu CC, et al. Baicalein inhibits HMGB1 release and MMP-2/-9 expression in lipopolysaccharide-induced cardiac hypertrophy. Am J Chin Med. 2014;42:785–97.
Zheng F, Wu J, Zhao S, Luo Q, Tang Q, Yang L, et al. Baicalein increases the expression and reciprocal interplay of RUNX3 and FOXO3a through crosstalk of AMPKalpha and MEK/ERK1/2 signaling pathways in human non-small cell lung cancer cells. J Exp Clin Cancer Res. 2015;34:41.
Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest. 2009;119:2758–71.
Murtaza I, Wang HX, Feng X, Alenina N, Bader M, Prabhakar BS, et al. Down-regulation of catalase and oxidative modification of protein kinase CK2 lead to the failure of apoptosis repressor with caspase recruitment domain to inhibit cardiomyocyte hypertrophy. J Biol Chem. 2008;283:5996–6004.
Wang K, Lin ZQ, Long B, Li JH, Zhou J, Li PF. Cardiac hypertrophy is positively regulated by MicroRNA miR-23a. J Biol Chem. 2012;287:589–99.
Wang JX, Li Q, Li PF. Apoptosis repressor with caspase recruitment domain contributes to chemotherapy resistance by abolishing mitochondrial fission mediated by dynamin-related protein-1. Cancer Res. 2009;69:492–500.
Liu CY, Zhang YH, Li RB, Zhou LY, An T, Zhang RC, et al. LncRNA CAIF inhibits autophagy and attenuates myocardial infarction by blocking p53-mediated myocardin transcription. Nat Commun. 2018;9:29.
Wang K, Long B, Liu F, Wang JX, Liu CY, Zhao B, et al. A circular RNA protects the heart from pathological hypertrophy and heart failure by targeting miR-223. Eur Heart J. 2016;37:2602–11.
Lin Z, Murtaza I, Wang K, Jiao J, Gao J, Li PF. MiR-23a functions downstream of NFATc3 to regulate cardiac hypertrophy. Proc Natl Acad Sci USA. 2009;106:12103–8.
Cui G, Luk SC, Li RA, Chan KK, Lei SW, Wang L, et al. Cytoprotection of baicalein against oxidative stress-induced cardiomyocytes injury through the Nrf2/Keap1 pathway. J Cardiovasc Pharmacol. 2015;65:39–46.
Li J, Chang WT, Li CQ, Lee C, Huang HH, Hsu CW, et al. Baicalein preventive treatment confers optimal cardioprotection by PTEN/Akt/NO activation. Am J Chin Med. 2017;45:987–1001.
Chang WT, Li J, Haung HH, Liu H, Han M, Ramachandran S, et al. Baicalein protects against doxorubicin-induced cardiotoxicity by attenuation of mitochondrial oxidant injury and JNK activation. J Cell Biochem. 2011;112:2873–81.
Li Z, Song Y, Liu L, Hou N, An X, Zhan D, et al. MiR-199a impairs autophagy and induces cardiac hypertrophy through mTOR activation. Cell Death Differ. 2017;24:1205–13.
Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–24.
Simonson B, Subramanya V, Chan MC, Zhang A, Franchino H, Ottaviano F. DDiT4L promotes autophagy and inhibits pathological cardiac hypertrophy in response to stress. Sci Signal 2017;10:eaaf5967.
Zhang W, Siraj S, Zhang R, Chen Q. Mitophagy receptor FUNDC1 regulates mitochondrial homeostasis and protects the heart from I/R injury. Autophagy. 2017;13:1080–1.
Li Y, Liu Z, Zhang Y, Zhao Q, Wang X, Lu P, et al. PEDF protects cardiomyocytes by promoting FUNDC1mediated mitophagy via PEDF-R under hypoxic condition. Int J Mol Med. 2018;41:3394–404.
Chen Y, Luo HQ, Sun LL, Xu MT, Yu J, Liu LL, et al. Dihydromyricetin attenuates myocardial hypertrophy induced by transverse aortic constriction via oxidative stress inhibition and SIRT3 pathway enhancement. Int J Mol Sci. 2018;19:2592.
Tan WQ, Wang K, Lv DY, Li PF. Foxo3a inhibits cardiomyocyte hypertrophy through transactivating catalase. J Biol Chem. 2008;283:29730–9.
Oka T, Akazawa H, Naito AT, Komuro I. Angiogenesis and cardiac hypertrophy: maintenance of cardiac function and causative roles in heart failure. Circ Res. 2014;114:565–71.
Zhao F, Fu L, Yang W, Dong Y, Yang J, Sun S, et al. Cardioprotective effects of baicalein on heart failure via modulation of Ca2+ handling proteins in vivo and in vitro. Life Sci. 2016;145:213–23.
Huang HH, Shao ZH, Li CQ, Vanden Hoek TL, Li J. Baicalein protects cardiomyocytes against mitochondrial oxidant injury associated with JNK inhibition and mitochondrial Akt activation. Am J Chin Med. 2014;42:79–94.
Yarla NS, Bishayee A, Sethi G, Reddanna P, Kalle AM, Dhananjaya BL, et al. Targeting arachidonic acid pathway by natural products for cancer prevention and therapy. Semin Cancer Biol. 2016;40–41:48–81.
Nho RS, Hergert P. FoxO3a and disease progression. World J Biol Chem. 2014;5:346–54.
Quotti Tubi L, Canovas Nunes S, Brancalion A, Doriguzzi Breatta E, Manni S, Mandato E, et al. Protein kinase CK2 regulates AKT, NF-kappaB and STAT3 activation, stem cell viability and proliferation in acute myeloid leukemia. Leukemia. 2017;31:292–300.
Das TP, Suman S, Alatassi H, Ankem MK, Damodaran C. Inhibition of AKT promotes FOXO3a-dependent apoptosis in prostate cancer. Cell Death Dis. 2016;7:e2111.
Marinkovic D, Zhang X, Yalcin S, Luciano JP, Brugnara C, Huber T, et al. Foxo3 is required for the regulation of oxidative stress in erythropoiesis. J Clin Invest. 2007;117:2133–44.
Guo L, Yin A, Zhang Q, Zhong T, O’Rourke ST, Sun C. Angiotensin-(1-7) attenuates angiotensin II-induced cardiac hypertrophy via a Sirt3-dependent mechanism. Am J Physiol Heart Circ Physiol. 2017;312:H980–91.
Wang C, Xu W, Zhang Y, Zhang F, Huang K. PARP1 promote autophagy in cardiomyocytes via modulating FoxO3a transcription. Cell Death Dis. 2018;9:1047.
Xiao Y, Yang Z, Wu QQ, Jiang XH, Yuan Y, Chang W, et al. Cucurbitacin B protects against pressure overload induced cardiac hypertrophy. J Cell Biochem. 2017;118:3899–910.
Guan XH, Hong X, Zhao N, Liu XH, Xiao YF, Chen TT, et al. CD38 promotes angiotensin II-induced cardiac hypertrophy. J Cell Mol Med. 2017;21:1492–502.
Meng G, Liu J, Liu S, Song Q, Liu L, Xie L, et al. Hydrogen sulfide pretreatment improves mitochondrial function in myocardial hypertrophy via a SIRT3-dependent manner. Br J Pharmacol. 2018;175:1126–45.
Chen B, Wu Q, Xiong Z, Ma Y, Yu S, Chen D, et al. Adenosine monophosphate-activated protein kinase attenuates cardiomyocyte hypertrophy through regulation of FOXO3a/MAFbx signaling pathway. Acta Biochim Biophys Sin. 2016;48:827–32.
Hauck L, Harms C, Grothe D, An J, Gertz K, Kronenberg G, et al. Critical role for FoxO3a-dependent regulation of p21CIP1/WAF1 in response to statin signaling in cardiac myocytes. Circ Res. 2007;100:50–60.
Chen M, Chen Z, Wang Y, Tan Z, Zhu C, Li Y, et al. Mitophagy receptor FUNDC1 regulates mitochondrial dynamics and mitophagy. Autophagy. 2016;12:689–702.
Liu L, Feng D, Chen G, Chen M, Zheng Q, Song P, et al. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat Cell Biol. 2012;14:177–85.
Facundo H, Brainard RE, Caldas FRL, Lucas AMB. Mitochondria and cardiac hypertrophy. Adv Exp Med Biol. 2017;982:203–26.
Dai DF, Johnson SC, Villarin JJ, Chin MT, NievesCintron M, Chen T, et al. Mitochondrial oxidative stress mediates angiotensin II-induced cardiac hypertrophy and Galphaq overexpression-induced heart failure. Circ Res. 2011;108:837–46.
Yu W, Xu M, Zhang T, Zhang Q, Zou C. Mst1 promotes cardiac ischemia-reperfusion injury by inhibiting the ERK-CREB pathway and repressing FUNDC1-mediated mitophagy. J Physiol Sci. 2019;69:113–27.
Weng LQ, Zhang WB, Ye Y, Yin PP, Yuan J, Wang XX, et al. Aliskiren ameliorates pressure overload-induced heart hypertrophy and fibrosis in mice. Acta Pharmacol Sin. 2014;35:1005–14.
Yin X, Peng C, Ning W, Li C, Ren Z, Zhang J, et al. MiR-30a downregulation aggravates pressure overload-induced cardiomyocyte hypertrophy. Mol Cell Biochem. 2013;379:1–6.
Pan W, Zhong Y, Cheng C, Liu B, Wang L, Li A, et al. MiR-30-regulated autophagy mediates angiotensin II-induced myocardial hypertrophy. PLoS ONE. 2013;8:e53950.
Acknowledgements
This work was supported by the Natural Science Foundation of Shandong Province (No. JQ201815), the National Natural Science Foundation of China (No. 81770232), and grants from FuWai Hospital (No. 2019kf-03).
Author information
Authors and Affiliations
Contributions
JXW and LL designed the study. BYL performed the experiments with help from GLL, WD, TX, XYJ, XXZ, and JZ. GLL, WD and TX contributed to the animal experiments, cell experiments, and data analysis. XYJ and XXZ contributed to the animal experiments. JZ contributed to the data analysis. BYL wrote the final manuscript. JXW and WGC revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Liu, By., Li, L., Liu, Gl. et al. Baicalein attenuates cardiac hypertrophy in mice via suppressing oxidative stress and activating autophagy in cardiomyocytes. Acta Pharmacol Sin 42, 701–714 (2021). https://doi.org/10.1038/s41401-020-0496-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-020-0496-1
Keywords
This article is cited by
-
Autophagy-related biomarkers in preeclampsia: the underlying mechanism, correlation to the immune microenvironment and drug screening
BMC Pregnancy and Childbirth (2024)
-
Lipoamide Attenuates Hypertensive Myocardial Hypertrophy Through PI3K/Akt-Mediated Nrf2 Signaling Pathway
Journal of Cardiovascular Translational Research (2024)
-
Malvidin alleviates LPS-induced septic intestinal injury through the nuclear factor erythroid 2-related factor 2/reactive oxygen species/NLRP3 inflammasome pathway
Inflammopharmacology (2024)
-
Nitidine chloride induces cardiac hypertrophy in mice by targeting autophagy-related 4B cysteine peptidase
Acta Pharmacologica Sinica (2023)
-
The mitophagy pathway and its implications in human diseases
Signal Transduction and Targeted Therapy (2023)