Abstract
Previous studies demonstrated that prolonged exposure to elevated levels of free fatty acids (FFA), especially saturated fatty acids, could lead to pancreatic β-cell apoptosis, which plays an important role in the progression of type 2 diabetes (T2D). Diacylglycerol acyltransferase 1 (DGAT1), an enzyme that catalyzes the final step of triglyceride (TG) synthesis, has been reported as a novel target for the treatment of multiple metabolic diseases. In this study we evaluated the potential beneficial effects of DGAT1 inhibitors on pancreatic β-cells, and further verified their antidiabetic effects in db/db mice. We showed that DGAT1 inhibitors (4a and LCQ908) at the concentration of 1 μM significantly ameliorated palmitic acid (PA)-induced apoptosis in MIN6 pancreatic β-cells and primary cultured mouse islets; oral administration of a DGAT1 inhibitor (4a) (100 mg/kg) for 4 weeks significantly reduced the apoptosis of pancreatic islets in db/db mice. Meanwhile, 4a administration significantly decreased fasting blood glucose and TG levels, and improved glucose tolerance and insulin tolerance in db/db mice. Furthermore, we revealed that pretreatment with 4a (1 μM) significantly alleviated PA-induced intracellular lipid accumulation, endoplasmic reticulum (ER) stress, and proinflammatory responses in MIN6 cells, which might contribute to the protective effects of DGAT1 inhibitors on pancreatic β-cells. These findings provided a better understanding of the antidiabetic effects of DGAT1 inhibitors.
Similar content being viewed by others
Introduction
Type 2 diabetes (T2D) is a chronic, progressive metabolic disease that is mainly characterized by elevated blood glucose levels [1]. The disease results from both genetic and environmental risk factors, and it is widely agreed that obesity serves as one of the most conclusive contributors in the progression of T2D [2]. Under the condition of T2D accompanied by obesity, circulating free fatty acids (FFA) levels are markedly increased, while chronic exposure to elevated levels of FFA could eventually lead to β-cell apoptosis [3,4,5]. Since pancreatic β-cell failure has already been demonstrated to play a critical role in the development of T2D [6], the protection of pancreatic β-cells has emerged as one of the important therapeutic strategies for the treatment of obesity-related T2D.
FFA serves pleiotropic functions in multiple physiological processes in pancreatic β-cells, including cell signaling, insulin secretion, membrane integrity, and energy metabolism [7]. Nevertheless, elevated circulating FFA has been considered as a major risk factor for obesity-related T2D. FFA overload could lead to apoptosis in several kinds of cells, such as pancreatic β-cells, hepatocytes, and adipocytes [8], and this apoptotic effect was verified to be strongly linked to mitochondrial dysfunction, endoplasmic reticulum (ER) stress, and inflammation [9,10,11].
Under normal physiological conditions, FFA is mainly packaged into triglycerides (TGs) and stored in adipose tissue. However, excess circulating FFA could lead to ectopic TG accumulation in nonadipose tissues [12], and several studies have indicated that lipid deposition could be responsible for the deleterious effects on pancreatic β-cells [13], and the reduction in TG could rescue β-cells from FFA-induced apoptosis [14].
The rate-limiting step of TG synthesis is mediated by acyl coenzyme A: diacylglycerol acyltransferases (DGATs), which catalyze the final esterification reaction with diacylglycerol and acyl-CoA as substrates [15]. In mammals, there are two identified DGAT isozymes, DGAT1 and DGAT2, and they both play crucial roles in lipid metabolism [16, 17]. While DGAT2-deficient mice died soon after birth due to lipopenia and skin barrier abnormalities [18], DGAT1-knockout mice were found to possess multiple beneficial characteristics, including enhanced energy expenditure and improved insulin and leptin sensitivity [19, 20]. Accordingly, DGAT1 has been regarded as a novel target for the treatment of T2D and disorders of lipid metabolism [21, 22]. In previous studies, overexpression of DGAT1 in isolated rat islets was verified to inhibit glucose-induced insulin secretion [23], and the glucose-dependent biosynthesis of neutral lipids catalyzed by DGAT was reported to cause lipotoxicity in pancreatic β-cells [24]. These results indicated that DGAT1 might be closely related to β-cell failure. However, little is known about the effect of DGAT1 inhibition on pancreatic β-cells, and the underlying mechanisms of DGAT1 inhibitors against T2D have not yet been fully ascertained.
The aim of this study was to investigate whether pharmaceutical inhibition of DGAT1 could serve any beneficial effects on pancreatic β-cells and to further confirm the antidiabetic effects of DGAT1 inhibitor on obese diabetic db/db mice.
Materials and methods
Materials and reagents
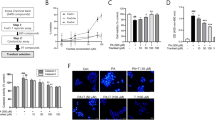
Roswell Park Memorial Institute (RPMI)-1640 medium, fetal bovine serum (FBS), and general reagents for cell culture were purchased from HyClone (Logan, UT, USA). Dulbecco’s modified Eagle’s medium (DMEM) and β-mercaptoethanol were obtained from Gibco (Carlsbad, CA, USA). Palmitic acid (PA), bovine serum albumin (BSA), Hoechst 33342, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), and collagenase V were purchased from Sigma-Aldrich (St. Louis, MO, USA). DGAT1 inhibitors 4a (purity > 98.0%, Fig. 1a) and LCQ908 (purity > 99.0%, Fig. 1b) were synthesized by Professor You-hong Hu’s group at the Shanghai Institute of Materia Medica, Chinese Academy of Sciences. The compounds were dissolved in dimethyl sulfoxide (DMSO), and the final concentration of DMSO in the culture medium was <0.1%.
The chemical structures of 4a and LCQ908 (a, b). MIN6 cells were pretreated with DGAT1 inhibitor (4a or LCQ908) for 1 h prior to treatment with 0.4 mM PA or vehicle for 48 h. Cell viability was detected by the MTT assay (c, d) (n = 5). All data are expressed as the mean ± SEM. Asterisks denote significant differences versus the PA-treated alone group (*P < 0.05, ***P < 0.001).
Cell culture and isolation of primary mouse pancreatic islets
Primary mouse pancreatic islets were isolated from 6-week-old male C57BL/6J mice as previously described [25]. Primary mouse pancreatic islets were maintained in RPMI-1640 medium with 10% FBS, 100 units/mL penicillin, and 100 mg/mL streptomycin (Invitrogen, Carlsbad, CA, USA) for 24 h before treatments. The mouse pancreatic β-cell line MIN6 cells were cultured in DMEM supplemented with 15% FBS and 50 μmol/L β-mercaptoethanol. MIN6 cells were passaged every 2 days, and cells at 15–25 passages were used for experiments. All cells were kept at 37 °C in a humidified atmosphere with 5% CO2.
Animal experiment
Male C57BL/KSJ-db/db mice (Jackson Laboratory, Bar Harbor, ME, USA) were bred and raised in the Shanghai Institute of Materia Medica. After 1 week of acclimatization, 6-week-old mice (35–40 g) were randomly divided into three groups (n = 8) and treated with DGAT1 inhibitor 4a (30 and 100 mg/kg) or vehicle (0.5% Tween-80) daily via oral administration. The lean littermates were treated with vehicle as a normal control. The oral glucose tolerance test was performed after 3 weeks of 4a administration. After overnight fasting, the mice were challenged with 0.8 g/kg glucose, and the blood glucose levels were examined at 0, 30, 60, 90, and 120 min. The insulin tolerance test was carried out after 4 weeks of 4a administration. Mice were fasted for 6 h and then intraperitoneally injected with 0.8 IU/kg insulin (from bovine pancreas), and the blood glucose levels were examined at 0, 30, 60, 90, 120, and 150 min. The glucose curves were recorded, and the areas under the curve were calculated. After 4 weeks of treatment, animals were subjected to isoflurane inhalation anesthesia, and then blood samples and tissues were collected for further analysis. All the experimental processes were approved by the Institutional Animal Care and Use Committee at the Shanghai Institute of Materia Medica, Chinese Academy of Sciences (permit number: 2015-01-WHY-13).
Cell viability assay
Cell viability was measured by the MTT assay. PA-BSA solution was prepared as previously reported [26]. MIN6 cells were treated with DGAT1 inhibitors in the presence or absence of 0.4 mM PA for 48 h. The cells were then incubated with MTT solution (0.5 mg/mL) at 37 °C for 4 h, and the absorbance at 492 nm was measured by the FlexStation 3 (Molecular Devices, San Jose, CA, USA).
Hoechst 33342 staining
MIN6 cells were seeded on cover glasses in a 24-well plate and were treated with DGAT1 inhibitors in the presence or absence of 0.4 mM PA for 48 h. Then, the cells were fixed in 4% paraformaldehyde, stained with Hoechst 33342 dye, and photographed by fluorescence microscopy (IX 73, Olympus, Tokyo, Japan).
Nile red staining and TG content determination
MIN6 cells were treated with DGAT1 inhibitors in the presence or absence of 0.4 mM PA for 48 h and then subjected to Nile red staining or intracellular TG determination. MIN6 cells were fixed with 4% paraformaldehyde for 15 min and subsequently stained with Nile red (Sigma-Aldrich) for 30 min and then photographed. TG content determination was performed with a TG assay kit (Ming Dian, Shanghai, China), and protein concentration was detected by a BCA protein kit (Beyotime, Shanghai, China). The cellular TG content was expressed as mmol/mg protein.
TUNEL staining
DNA strand breaks in apoptotic cells were detected by a terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay. Isolated mouse islets were treated with the DGAT1 inhibitor 4a in the presence or absence of 0.4 mM PA for 48 h, and apoptotic cells were detected by a TUNEL kit (Roche, Mannheim, Germany) according to the manufacturer’s instructions. Then, the cells were stained with Hoechst 33342, and photos were taken by confocal microscopy (FV10i, Olympus, Tokyo, Japan).
Immunofluorescence assay
Immunofluorescence staining was performed as described previously [27]. The pancreas was fixed in 4% paraformaldehyde, and the tissues were embedded in paraffin and sectioned. After deparaffinization and washing, pancreas sections were subjected to TUNEL staining and then incubated with primary antibodies (rabbit anti-insulin antibody, dilution 1:100, Cell Signaling Technology, Danvers, MA, USA) and secondary antibodies (Alexa Fluor 488-conjugated goat anti-rabbit IgG, dilution 1:400, Invitrogen). After Hoechst 33342 staining, pancreas sections were captured by fluorescence microscopy. The number of TUNEL-positive cells and total cells were determined.
Western blot analysis
MIN6 cells were treated with DGAT1 inhibitors in the presence or absence of 0.4 mM PA for 48 h, and total protein was extracted by RIPA lysis buffer (Beyotime) containing protease inhibitors (Sigma-Aldrich) and phosphatase inhibitors (Roche). The expression levels of proteins were detected by Western blot analysis as previously described [28]. The following primary antibodies were used in this study: cleaved caspase-3 (Asp175), protein kinase R-like endoplasmic reticulum kinase (PERK), phosphorylated-PERK, eukaryotic translation initiation factor 2 alpha (eIF2α), phosphorylated-eIF2α, c-Jun N-terminal kinase (JNK), phosphorylated-JNK, p38, phosphorylated-p38 (all from Cell Signaling Technology), and C/EBP homologous protein (CHOP) (Santa Cruz Biotechnology, Dallas, TX, USA). The secondary antibodies were purchased from Jackson Laboratory. Relative expression levels of proteins were analyzed by optical density using ImageJ (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All data are presented as the mean ± SEM. All experiments were independently repeated at least three times. The comparison of different groups was assessed by unpaired Student’s t test or one-way ANOVA followed by the Tukey’s post hoc test. Differences were considered statistically significant at P < 0.05. GraphPad Prism 5 software (GraphPad Software, San Diego, CA, USA) was used for statistical analyses.
Results
DGAT1 inhibitors protect MIN6 β-cells from PA-induced apoptosis
Long-chain saturated fatty acids were demonstrated to have lipotoxic effects on pancreatic β-cells [3], and DGAT1-mediated lipid synthesis was reported to be closely related to FFA-induced lipotoxicity [24]. Since DGAT1 was verified to be expressed in MIN6 β-cells [29], a cell viability assay was performed to determine whether DGAT1 inhibitors could serve protective roles in PA-induced MIN6 cell injury. The results showed that compared with the PA-treated alone group, DGAT1 inhibitors 4a (A 922500) and LCQ908 (Pradigastat) (Fig. 1a, b) dose-dependently increased cell viability (Fig. 1c, d). To further evaluate the protective effects of DGAT1 inhibitors on MIN6 cells, Hoechst 33342 staining was carried out to examine the apoptotic rate. Highly condensed chromatin, one of the most convincing symbols of cell apoptosis, was extensively observed in PA-treated cells, while both 4a and LCQ908 significantly and dose-dependently decreased the apoptotic rate of MIN6 cells in the presence of PA (Fig. 2a, b). Western blot analysis also revealed that the expression of cleaved caspase-3, the key executor of cell apoptosis, was downregulated by 4a and LCQ908 in a dose-dependent manner (Fig. 2c). These results illustrated that the DGAT1 inhibitors could protect MIN6 cells from PA-induced apoptosis.
MIN6 cells were pretreated with DGAT1 inhibitors (4a or LCQ908) for 1 h prior to treatment with 0.4 mM PA or vehicle for 48 h. The apoptosis rate was detected by Hoechst 33342 staining, and the apoptotic cells are indicated by arrows (n = 4, scale bar = 50 μm) (a, b). Expression of cleaved caspase-3 (c) was detected by Western blot analysis (n = 5). All data are expressed as the mean ± SEM. Asterisks denote significant differences versus the PA-treated alone group (*P < 0.05, **P < 0.01, ***P < 0.001).
4a ameliorates the apoptosis of mouse islet cells both in vitro and in vivo
To further verify the protective effect of the DGAT1 inhibitor on islet cells, TUNEL staining was performed to detect apoptotic cells by labeling DNA strand breaks in primary cultured mouse islets. After pretreatment with 4a and then exposure to PA for 48 h, the number of TUNEL-positive apoptotic cells dramatically increased in PA-treated isolated islets, while 4a was found to significantly inhibit the detrimental effect caused by PA (Fig. 3a, b). Likewise, a similar conclusion was also obtained from the in vivo experiment. Long-term oral administration of 4a reduced the apoptosis rate of islet cells in db/db mice (Fig. 3c, d). These data corroborated that the DGAT1 inhibitor 4a was able to protect islet cells from apoptosis both in vitro and in vivo.
Isolated mouse islets were pretreated with DGAT1 inhibitor 4a for 1 h prior to treatment with 0.4 mM PA or vehicle for 48 h. Then, TUNEL staining was carried out, and the number of apoptotic cells (indicated by arrows) was counted (n = 5, scale bar = 50 μm) (a, b). After 4 weeks of oral administration of 4a or vehicle, TUNEL staining and immunofluorescence assays were performed to detect apoptotic cells (indicated by arrows) in the islets of db/db mice (n = 4, scale bar = 50 μm) (c), and the apoptosis rate was then calculated (d). All data were expressed as the mean ± SEM. Asterisks denote significant differences versus the PA-treated alone group or the vehicle-treated group (*P < 0.05, **P < 0.01).
Long-term administration of 4a improves glucose and lipid metabolism in db/db mice
Since improved insulin sensitivity and decreased lipid accumulation were observed in mice lacking DGAT1 [23], we wondered whether DGAT1 inhibitors were capable of regulating the altered metabolic homeostasis in db/db mice. After 4 weeks of oral administration of 4a or vehicle, there were no significant differences in food intake among all the groups (data not shown), and 100 mg/kg 4a could considerably reduce body weight gain (Fig. 4a), which might be due to the inhibition of absorption and synthesis of TG. Fasting glucose and TG levels were both decreased (Fig. 4b, c), and oral glucose tolerance (Fig. 4d, e) and insulin tolerance (Fig. 4f, g) were obviously improved in mice treated with 100 mg/kg 4a. The plasma levels of total cholesterol and low-density lipoprotein cholesterol were not affected by 4a (data not shown). These results indicated that 4a could ameliorate glucose and lipid metabolism in db/db mice.
Body weights were monitored during the whole experiment, and body weight gain was then calculated (a). After 6 h of fasting, blood samples were collected, and the serum levels of glucose (b) and TG (c) were detected. OGTT and ITT were performed after 3 weeks and 4 weeks of 4a administration, respectively. Then, the glucose curves were recorded, and the areas under the curve (AUCs) were calculated (d–g) (C57, open circles; vehicle, filled squares; 30 mg/kg 4a, filled triangles; 100 mg/kg 4a, filled inverted triangles). All data are expressed as the mean ± SEM (n = 8). Asterisks denote significant differences versus the vehicle-treated group (*P < 0.05, **P < 0.01, ***P < 0.001).
4a inhibits PA-induced lipid accumulation, ER stress, and proinflammatory responses in MIN6 cells
We conjectured that the beneficial effects of DGAT1 inhibitors might be ascribed to the reduction in TG accumulation in pancreatic β-cells, thereby blocking the progression of apoptosis. Supported by the results of TG content determination and Nile Red staining, both 4a and LCQ908 could markedly inhibit lipid accumulation induced by PA in MIN6 cells (Fig. 5a, b). Meanwhile, to further investigate the possible mechanisms of the protective effects of DGAT1 inhibitors, we also detected several signaling pathways that were involved in PA-induced lipotoxicity. Western blot analysis showed that ER stress and inflammation pathways were markedly activated in PA-treated MIN6 cells. In the presence of PA, 4a was found to dose-dependently decrease the expression of proteins involved in ER stress, such as CHOP, phosphorylated PERK, and phosphorylated eIF2α (Fig. 5c, d), and PA-induced elevated levels of inflammation-related proteins, including phosphorylated JNK and phosphorylated p38, were also reduced in MIN6 cells when cotreated with 4a (Fig. 5e, f). These findings revealed that DGAT1 inhibitor 4a restrained PA-induced lipid accumulation, ER stress, and proinflammatory responses, which might help to protect pancreatic β-cells from PA-induced apoptosis.
MIN6 cells were pretreated with 4a or LCQ908 for 1 h prior to treatment with 0.4 mM PA or vehicle for 48 h, and then the TG content was determined (a). Intracellular neutral lipids were stained with Nile red (b) (scale bar = 50 μm). Western blotting was performed to analyze the expression of proteins related to ER stress (c, d) and inflammation (e, f). All data are expressed as the mean ± SEM (n = 3). Asterisks denote significant differences versus the PA-treated alone group (*P < 0.05, **P < 0.01, ***P < 0.001).
Discussion
DGAT1 catalyzes the rate-limiting step of TG synthesis and has been recognized as a potential therapeutic target for a variety of metabolic disorders, and several DGAT1 inhibitors have already entered clinical trials [22]. Although inhibition of DGAT1 has been reported to exert antidiabetic effects in several animal models [30,31,32], the mechanisms have not yet been completely elucidated. In this study, we identified that DGAT1 inhibitors could exert antiapoptotic effects on pancreatic β-cells under chronic FFA-overloaded conditions both in vitro and in vivo, and the possible underlying mechanisms were also discussed. We also confirmed that 4a was capable of ameliorating glucose and lipid metabolism in obese diabetic db/db mice. These findings indicated a new effect of DGAT1 inhibitors, which could provide novel insights into the protection of pancreatic β-cells.
FFA has been confirmed to play essential roles in maintaining the normal physiological functions of pancreatic β-cells, but excessive FFA could impair β-cell functions and result in β-cell apoptosis [1]. It was also demonstrated that elevated FFA was responsible for ectopic lipid deposition in the pancreas, which was recently identified as a possible predictor of T2D [13]. Lipotoxicity was suggested to be closely related to the carbon chain length and the saturation degree of FFA. Prolonged exposure to saturated FFA, such as PA, was found to cause apoptosis and decrease the gene expression of insulin in β-cells [33]. Not surprisingly, we found that long-term exposure of MIN6 cells to PA could dramatically decrease cell viability and increase the apoptotic rate, while DGAT1 inhibitors were able to dose-dependently reduce the adverse effect and protect MIN6 cells from apoptosis in the presence of PA. Likewise, the protective role of 4a was also confirmed in PA-treated primary cultured mouse islets and the pancreatic islets of obese diabetic db/db mice.
Since an oversupply of FFA could trigger apoptosis in β-cells, a host of work has been done to determine the underlying mechanisms. A previous study indicated an inverse relationship between cellular TG accumulation and FFA-induced cytotoxicity in pancreatic islets [34]. However, a reduction in TG was reported to block the FFA-induced apoptosis and restore β-cell function in islets isolated from Zucker diabetic fatty rats [14, 35]. Another study also discovered that kaempferol, a natural flavonoid, could reduce lipid accumulation through lipophagy, thus preserving β-cell function in RIN-5F β-cells [36]. In addition, ER stress was verified to participate in FFA-induced lipotoxicity. PA was reported to interfere with ER-to-Golgi trafficking and eventually lead to ER stress in MIN6 and INS-1 cells [37]. PA was also verified to cause tripalmitin accumulation in the ER of β-cells [3], and a recent study reported that inhibition of lipid export from the ER could lead to TG accumulation in the ER and exacerbate ER stress at the same time [38]. Prolonged ER stress could eventually lead to β-cell apoptosis, which was induced by the activation of ER stress downstream signaling pathways, such as CHOP [39, 40]. Meanwhile, PA-induced apoptosis was also reported to have a strong connection with inflammation-related signaling pathways, including JNK and p38 [11]. In this study, DGAT1 inhibitors inhibited intracellular lipid accumulation and downregulated the expression of proteins involved in ER stress and inflammation pathways in MIN6 cells. We thus preliminarily confirmed that the protective effects of DGAT1 inhibitors on β-cells might be attributed to the suppression of lipid deposition and the amelioration of ER stress and proinflammatory responses.
Several studies have indicated that DGAT1 inhibition has multiple beneficial effects on metabolic diseases. As reported, DGAT1-deficient mice showed beneficial metabolic phenotypes, such as prolonged release of gut GLP-1 and delayed gastric emptying [41], and DGAT1 knockout was proved to significantly enhance insulin and leptin sensitivity [20]. In addition, long-term oral administration of a DGAT1 inhibitor was also demonstrated to improve insulin sensitivity in DIO mice [42]. Here, we verified that 4a suppressed body weight gain and reduced the circulating levels of glucose and TG in obese diabetic db/db mice at a dose of 100 mg/kg, while there were no differences in food intake among all the groups. Moreover, glucose tolerance and insulin tolerance were improved at the same time. These results revealed that the DGAT1 inhibitor 4a markedly ameliorated glucose and lipid metabolism in db/db mice.
The beneficial role of DGAT1 inhibition has been confirmed in several animal models, whereas DGAT1 inhibition was also inferred to exert diverse effects on specific tissues. A recent study showed that DGAT1-mediated fatty acid reesterification could protect the ER from lipotoxicity in white adipose tissue (WAT), while DGAT1 deficiency could augment the unfolded protein response in WAT and cause inflammation in macrophages [43]. Several studies have also shown that DGAT1 overexpression in skeletal muscle could promote TG synthesis and subsequently prevent high-fat diet-induced insulin resistance, while DGAT1 deficiency was shown to impair insulin sensitivity in isolated muscle [44, 45]. Moreover, overexpression of DGAT1 was claimed to aggravate lipotoxicity in pancreatic β-cells [23], but no correlated studies have been reported to investigate the effects of pharmaceutical inhibition or knockout of DGAT1 on β-cells. Here, we validated that DGAT1 inhibitors were able to exert antiapoptotic effects on lipid-overloaded β-cells, and we speculated that the excess FFA might be channeled into other pools and participate in multiple physiological processes, such as lipid oxidation and biomembrane synthesis. Therefore, DGAT1 inhibitors with better tissue distribution patterns might be developed to maximize the beneficial effects and minimize the tissue-specific adverse effects at the same time. Nevertheless, this study only disclosed the beneficial effects of DGAT1 inhibitors, the role of DGAT1 in β-cells and the detailed mechanisms still need to be thoroughly investigated, and β-cell-specific DGAT1-knockout experiments should also be carried out. In addition, β-cell dysfunction also plays an important role in the progression of T2D, and it is absolutely indispensable to further explore the effects of DGAT1 inhibitors on β-cell functions under FFA-overloaded conditions, including insulin synthesis and secretion.
In conclusion, this study demonstrated that DGAT1 inhibitors could reduce PA-induced intracellular TG accumulation, ER stress, and proinflammatory responses, which might contribute to the antiapoptotic effect on pancreatic β-cells. These beneficial effects might provide a better understanding of the roles of DGAT1 inhibitors in the prevention and treatment of T2D, and our findings suggested that the combination of DGAT1 inhibitors and other antidiabetic drugs might show better efficacy in improving glucose and lipid metabolism at the same time.
References
Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. 2003;46:3–19.
Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nat Rev Endocrinol. 2011;8:228–36.
Moffitt JH, Fielding BA, Evershed R, Berstan R, Currie JM, Clark A. Adverse physicochemical properties of tripalmitin in beta cells lead to morphological changes and lipotoxicity in vitro. Diabetologia. 2005;48:1819–29.
Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008;29:351–66.
Unger RH. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM: genetic and clinical implications. Diabetes. 1995;44:863–70.
Soumaya K. Molecular mechanisms of insulin resistance in diabetes. In: Ahmad SI, editor. Diabetes: an old disease, a new insight. New York (NY): Springer; 2013. p. 240–51.
Boucher A, Lu D, Burgess SC, Telemaque-Potts S, Jensen MV, Mulder H, et al. Biochemical mechanism of lipid-induced impairment of glucose-stimulated insulin secretion and reversal with a malate analogue. J Biol Chem. 2004;279:27263–71.
Kusminski CM, Shetty S, Orci L, Unger RH, Scherer PE. Diabetes and apoptosis: lipotoxicity. Apoptosis. 2009;14:1484–95.
Janikiewicz J, Hanzelka K, Kozinski K, Kolczynska K, Dobrzyn A. Islet β-cell failure in type 2 diabetes – within the network of toxic lipids. Biochem Biophys Res Commun. 2015;460:491–6.
Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–7.
Liu J, Chang F, Li F, Fu H, Wang J, Zhang S, et al. Palmitate promotes autophagy and apoptosis through ROS-dependent JNK and p38 MAPK. Biochem Biophys Res Commun. 2015;463:262–7.
Després J-P, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28:1039–49.
Lee Y, Lingvay I, Szczepaniak LS, Ravazzola M, Orci L, Unger RH. Pancreatic steatosis: harbinger of type 2 diabetes in obese rodents. Int J Obes. 2009;34:396–400.
Shimabukuro M, Zhou YT, Levi M, Unger RH. Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci USA. 1998;95:2498–502.
Shi Y, Cheng D. Beyond triglyceride synthesis: the dynamic functional roles of MGAT and DGAT enzymes in energy metabolism. Am J Physiol Endocrinol Metab. 2009;297:E10–8.
Bhatt-Wessel B, Jordan TW, Miller JH, Peng L. Role of DGAT enzymes in triacylglycerol metabolism. Arch Biochem Biophys. 2018;655:1–11.
Yen C-LE, Stone SJ, Koliwad S, Harris C, Farese RV Jr. Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J Lipid Res. 2008;49:2283–301.
Stone SJ, Myers HM, Watkins SM, Brown BE, Feingold KR, Elias PM, et al. Lipopenia and skin barrier abnormalities in DGAT2-deficient mice. J Biol Chem. 2004;279:11767–76.
Chen HC, Ladha Z, Smith SJ, Robert V, Farese J. Analysis of energy expenditure at different ambient temperatures in mice lacking DGAT1. Am J Physiol Endocrinol Metab. 2003;284:E213–8.
Chen HC, Smith SJ, Ladha Z, Jensen DR, Ferreira LD, Pulawa LK, et al. Increased insulin and leptin sensitivity in mice lacking acyl CoA:diacylglycerol acyltransferase 1. J Clin Invest. 2002;109:1049–55.
Ohshiro T, Tomoda H. Acyltransferase inhibitors: a patent review (2010–present). Expert Opin Ther Pat. 2015;25:145–58.
Zammit VA, Buckett LK, Turnbull AV, Wure H, Proven A. Diacylglycerol acyltransferases: potential roles as pharmacological targets. Pharmacol Ther. 2008;118:295–302.
Kelpe CL, Johnson LM, Poitout V. Increasing triglyceride synthesis inhibits glucose-induced insulin secretion in isolated rat islets of langerhans: a study using adenoviral expression of diacylglycerol acyltransferase. Endocrinology. 2002;143:3326–32.
Briaud I, Harmon JS, Kelpe CL, Segu VB, Poitout V. Lipotoxicity of the pancreatic beta-cell is associated with glucose-dependent esterification of fatty acids into neutral lipids. Diabetes. 2001;50:315–21.
Wang T, Sun P, Chen L, Huang Q, Chen K, Jia Q, et al. Cinnamtannin D-1 protects pancreatic beta-cells from palmitic acid-induced apoptosis by attenuating oxidative stress. J Agric Food Chem. 2014;62:5038–45.
Martinez SC, Tanabe K, Cras-Meneur C, Abumrad NA, Bernal-Mizrachi E, Permutt MA. Inhibition of Foxo1 protects pancreatic islet beta-cells against fatty acid and endoplasmic reticulum stress-induced apoptosis. Diabetes. 2008;57:846–59.
Wu J, Sun P, Zhang X, Liu H, Jiang H, Zhu W, et al. Inhibition of GPR40 protects MIN6 beta cells from palmitate-induced ER stress and apoptosis. J Cell Biochem. 2012;113:1152–8.
Hu H, He LY, Gong Z, Li N, Lu YN, Zhai QW, et al. A novel class of antagonists for the FFAs receptor GPR40. Biochem Biophys Res Commun. 2009;390:557–63.
Thorn K, Bergsten P. Fatty acid-induced oxidation and triglyceride formation is higher in insulin-producing MIN6 cells exposed to oleate compared to palmitate. J Cell Biochem. 2010;111:497–507.
Dow RL, Andrews MP, Li JC, Michael Gibbs E, Guzman-Perez A, LaPerle JL, et al. Defining the key pharmacophore elements of PF-04620110: discovery of a potent, orally-active, neutral DGAT-1 inhibitor. Bioorg Med Chem. 2013;21:5081–97.
Kwak HJ, Pyun YM, Kim JY, Pagire HS, Kim KY, Kim KR, et al. Synthesis and biological evaluation of aminobenzimidazole derivatives with a phenylcyclohexyl acetic acid group as anti-obesity and anti-diabetic agents. Bioorg Med Chem Lett. 2013;23:4713–8.
Tomimoto D, Okuma C, Ishii Y, Akiyama Y, Ohta T, Kakutani M. et al. Pharmacological characterization of [trans-5'-(4-amino-7,7-dimethyl-2-trifluoromethyl-7H-pyrimido[4,5-b][1,4]oxazin-6-yl)-2',3'-dihydrospiro(cyclohexane-1,1'-inden)-4-yl]acetic acid monobenzenesulfonate(JTT-553), a novel acyl CoA:diacylglycerol transferase (DGAT) 1 inhibitor. Biol Pharm Bull. 2015;38:263–9.
Hagman DK, Hays LB, Parazzoli SD, Poitout V. Palmitate inhibits insulin gene expression by altering PDX-1 nuclear localization and reducing MafA expression in isolated rat islets of Langerhans. J Biol Chem. 2005;280:32413–8.
Cnop M, Hannaert JC, Hoorens A, Eizirik DL, Pipeleers DG. Inverse relationship between cytotoxicity of free fatty acids in pancreatic islet cells and cellular triglyceride accumulation. Diabetes. 2001;50:1771–7.
Shimabukuro M, Zhou YT, Lee Y, Unger RH. Troglitazone lowers islet fat and restores beta cell function of Zucker diabetic fatty rats. J Biol Chem. 1998;273:3547–50.
Varshney R, Varshney R, Mishra R, Gupta S, Sircar D, Roy P. Kaempferol alleviates palmitic acid-induced lipid stores, endoplasmic reticulum stress and pancreatic beta-cell dysfunction through AMPK/mTOR-mediated lipophagy. J Nutr Biochem. 2018;57:212–27.
Preston AM, Gurisik E, Bartley C, Laybutt DR, Biden TJ. Reduced endoplasmic reticulum (ER)-to-Golgi protein trafficking contributes to ER stress in lipotoxic mouse beta cells by promoting protein overload. Diabetologia. 2009;52:2369–73.
Gu Y, Yang Y, Cao X, Zhao Y, Gao X, Sun C, et al. Plin3 protects against alcoholic liver injury by facilitating lipid export from the endoplasmic reticulum. J Cell Biochem. 2019;120:16075–87.
Fonseca SG, Gromada J, Urano F. Endoplasmic reticulum stress and pancreatic beta-cell death. Trends Endocrinol Metab. 2011;22:266–74.
Han J, Kaufman RJ. The role of ER stress in lipid metabolism and lipotoxicity. J Lipid Res. 2016;57:1329–38.
Okawa M, Fujii K, Ohbuchi K, Okumoto M, Aragane K, Sato H, et al. Role of MGAT2 and DGAT1 in the release of gut peptides after triglyceride ingestion. Biochem Biophys Res Commun. 2009;390:377–81.
Maciejewski BS, LaPerle JL, Chen D, Ghosh A, Zavadoski WJ, McDonald TS, et al. Pharmacological inhibition to examine the role of DGAT1 in dietary lipid absorption in rodents and humans. Am J Physiol Gastrointest Liver Physiol. 2013;304:G958–69.
Chitraju C, Mejhert N, Haas JT, Diaz-Ramirez LG, Grueter CA, Imbriglio JE, et al. Triglyceride synthesis by DGAT1 protects adipocytes from lipid-induced ER stress during lipolysis. Cell Metab. 2017;26:407–18.e3.
Liu L, Zhang Y, Chen N, Shi X, Tsang B, Yu YH. Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat-induced insulin resistance. J Clin Invest. 2007;117:1679–89.
Timmers S, de Vogel-van den Bosch J, Hesselink MK, van Beurden D, Schaart G, Ferraz MJ, et al. Paradoxical increase in TAG and DAG content parallel the insulin sensitizing effect of unilateral DGAT1 overexpression in rat skeletal muscle. PLoS One. 2011;6:e14503.
Acknowledgements
We gratefully acknowledge the kind provision of MIN6 cells by Professor S. Seino (Division of Molecular and Metabolic Medicine, Kobe University Graduate School of Medicine, Kobe, Japan). This work was supported by grants from the National Science & Technology Major Project “Key New Drug Creation and Manufacturing Program,” China (2018ZX09711002-002-007); the “Personalized Medicines-Molecular Signature-based Drug Discovery and Development,” the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA12040336); the National Natural Science Foundation of China (81503124, 81773791, and 81473262); and the Institutes for Drug Discovery and Development, Chinese Academy of Sciences (CASIMM0120162025).
Author information
Authors and Affiliations
Contributions
HYW and TW designed the research; JSH, BBG, and GHW performed the research and analyzed the data; LMZ and YHH synthesized the compounds; JSH, TW, and HYW wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Huang, Js., Guo, Bb., Wang, Gh. et al. DGAT1 inhibitors protect pancreatic β-cells from palmitic acid-induced apoptosis. Acta Pharmacol Sin 42, 264–271 (2021). https://doi.org/10.1038/s41401-020-0482-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-020-0482-7
Keywords
This article is cited by
-
Silencing of DIRAS3 improves the proliferation and insulin secretion of palmitic acid-treated pancreatic β‑cells through regulating PI3K/AKT signaling
International Journal of Diabetes in Developing Countries (2023)
-
Acacetin antagonized lipotoxicity in pancreatic β-cells via ameliorating oxidative stress and endoplasmic reticulum stress
Molecular Biology Reports (2022)