Abstract
Breast cancer resistance protein (BCRP) and P-glycoprotein (P-gp) are co-located at blood–brain barrier (BBB) cells, preventing their substrates from entering brain. Accumulating evidence demonstrates that liver failure impairs P-gp and BCRP expression and function in the brain. In the current study, we investigated how liver failure influenced the expression and function of brain BCRP and P-gp in rats subjected to bile duct ligation (BDL). The function of BCRP, P-gp and BBB integrity was assessed using distribution of prazosin, rhodamine 123 and fluorescein, respectively. We showed that BDL significantly decreased BCRP function, but increased P-gp function without affecting BBB integrity. Furthermore, we found that BDL significantly downregulated the expression of membrane BCRP and upregulated the expression of membrane P-gp protein in the cortex and hippocampus. In human cerebral microvascular endothelial cells, NH4Cl plus unconjugated bilirubin significantly decreased BCRP function and expression of membrane BCRP protein, but upregulated P-gp function and expression of membrane P-gp protein. The decreased expression of membrane BCRP protein was linked to the decreased expression of membrane radixin protein, while the increased expression of membrane P-gp protein was related to the increased location of membrane ezrin protein. Silencing ezrin impaired membrane location of P-gp, whereas silencing radixin impaired membrane location of BCRP protein. BDL rats showed the increased expression of membrane ezrin protein and decreased expression of membrane radixin protein in the brain. We conclude that BDL causes opposite effects on the expression and function of brain BCRP and P-gp, attributing to the altered expression of membrane radixin and ezrin protein, respectively, due to hyperbilirubinemia and hyperammonemia.
Similar content being viewed by others
Introduction
Hepatic encephalopathy (HE) is a neuropsychiatric complication of acute liver failure and chronic liver failure that results from dyshomeostasis of the central nervous system (CNS) due to the accumulation of neurotoxins or neuroactive substances in the brain [1,2,3]. Under physiological conditions, the maintenance of CNS homeostasis is highly dependent on the blood–brain barrier (BBB). The BBB maintains CNS homeostasis by preventing harmful substances from entering the brain from the blood or by efflux these toxins from the brain [4, 5]. The BBB also expresses some ATP-binding cassette (ABC) transporters, such as P-glycoprotein (P-gp), multidrug resistance-associated proteins (MRPs) and breast cancer resistance protein (BCRP), which are components of the BBB [3, 6]. The function of these ABC transporters is to efflux drugs or toxins from the CNS [7]. Our previous studies have demonstrated that liver injury markedly affects the function and expression of P-gp, BCRP and MRP2 in the BBB [8,9,10,11]. These changes are often dependent on the type of liver failure and the species of the ABC transporter [12]. In rodents with thioacetamide-induced liver failure, the function and expression of P-gp and BCRP in the brain were significantly decreased, but the function and expression of MRP2 were increased [9, 11]. Liver failure induced by bile duct ligation (BDL) impaired the function and expression of BCRP in the BBB of rats [8]. Liver failure is often characterized by hyperammonemia and hyperbilirubinemia [13,14,15,16]. The impairment of BCRP function and expression in the BBB due to the liver failure can be attributed to both hyperammonemia and hyperbilirubinemia [8, 10]. Ammonia impaired the expression and function of BCRP via the activation of the ROS-ERK1/2 pathway [10]. In rats, it was found that acute hyperammonemia induced by ammonium acetate increased rather than decreased the expression and function of P-gp and MRP2 in the brain [17], but this finding did not explain the decrease in P-gp function and expression in the BBB of rodents with thioacetamide-induced liver failure [9, 11]. Increased levels of bile acids, especially chenodeoxycholic acid, were also observed in the serum of rats suffering from liver injury, and these increased bile acid levels may decrease the function and expression of P-gp in the brain [11]. A high concentration of ammonia (5 mM) or acute hyperammonemia increased the function and expression of P-gp by activating the NF-κB pathway [14], but a low concentration of ammonia (1 mM) or chronic hyperammonemia mainly increased the membrane expression P-gp proteins partly via the activation of the ROS-ERK1/2 pathway [18]. BDL-induced liver failure is often accompanied by hyperammonemia, hyperbilirubinemia and hyperbileacidemia, indicating that the alterations in P-gp function and expression in the brains of BDL-treated rats are complicated. Our preliminary experiment showed that BDL decreased the function and total protein expression of BCRP as well as the membrane expression of the BCRP protein but increased the function of P-gp in the rat brain. More importantly, the total protein expression of P-gp was significantly decreased, but the membrane expression of the P-gp protein was markedly increased.
Ezrin/radixin/moesin (ERM) proteins function as general cross-linkers between plasma membrane proteins and the actin cytoskeleton and are involved in the functional expression of membrane proteins on the cell surface, including P-gp and BCRP [19,20,21,22,23]. The regulation of the functions of BCRP and P-gp by ERM proteins is often organ-specific. For example, in HepG2 cells [19], it was reported that the knockdown of ezrin decreased the mRNA levels of P-gp and that knockdown of radixin decreased the membrane expression of the P-gp protein; these results were consistent with the significant increase in the accumulation of rhodamine 123. Knockdown of moesin did not affect the expression or function of P-gp. In HCC827 cells, the efflux rates of SN-38 and rhodamine 123 were significantly decreased by knockdown of ezrin or moesin but not by knockdown of radixin. In Caco-2 cells, neither ezrin nor radixin knockdown affected BCRP function. Caco-2 cells do not express moesin. In Caki-1 cells, knockdown of radixin (but not ezrin or moesin) increased the intracellular SN-38 concentration [20].
The first aim of this study was to further confirm the opposite regulation of the functions of P-gp and BCRP in the brains of rats treated with BDL. BDL-induced liver failure is characterized by hyperammonemia and hyperbilirubinemia [8, 24]. The second aim was to investigate whether abnormally altered components (such as ammonia and bilirubin) in the serum of rats treated with BDL were factors that oppositely regulate the functions of P-gp and BCRP using human cerebral microvascular endothelial cells (HCMEC/D3 cells) as an in vitro model of the BBB. The final aim was to determine whether the opposite regulation of the membrane proteins P-gp and BCRP by BDL resulted from changes in the expression of ERM proteins. The results will reveal the mechanisms by which BDL oppositely regulates the functions of P-gp and BCRP in the BBB.
Materials and methods
Reagents
Prazosin hydrochloride was obtained from the National Institutes for Food and Drug Control (Beijing, China). Fluorescein sodium and rhodamine 123 were obtained from Aladdin Co., Ltd. (Shanghai, China). Kits to analyze the total bilirubin (TBIL), unconjugated bilirubin (UCB), total bile acids (TBAs), blood ammonia, alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP) levels were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Cell Counting Kit-8 (CCK-8) was obtained from Vazyme Biotech Co., Ltd. (Nanjing, China). The antibodies against BCRP, P-gp, ERM, ezrin, radixin, moesin, and CD71 and the HRP-conjugated secondary antibodies were obtained from Cell Signaling Technology (Danvers, MA, USA). The antibodies against β-actin were obtained from Bioworld (Louis Park, MN, USA). The human cerebral microvascular endothelial cell line HCMEC/D3 was obtained from JENNIO Biological Technology Ltd. (Guangzhou, China). RPMI-1640 medium, fetal bovine serum (FBS), Opti-MEM and Lipofectamine 3000 were obtained from Invitrogen (Waltham, CA, USA). All the other reagents were of analytical grade and were commercially available.
Animals and development of BDL rats
Male Sprague Dawley rats weighing 220–240 g from Super-B&K Experimental Animal Co., Ltd. (Shanghai, China) were housed under controlled environmental conditions (temperature of 25 ± 1 °C, humidity of 50% ± 5%) with 12-h light/dark cycles. After 7 days of acclimation, BDL was performed on the rats according to a previously described method [8, 24]. The sham operation (SHAM) rats underwent the same operations as the BDL rats except BDL. After 2 weeks, the experimental rats were used for the following experiments. All the experimental procedures and animal protocols were approved by the Animal Ethics Committee of China Pharmaceutical University.
Distribution of prazosin, rhodamine 123 and fluorescein in the rat brain
Cocktail probes consisting of prazosin (1 mg/kg), rhodamine 123(0.2 mg/kg) and fluorescein sodium (2 mg/kg) were intravenously administered to the rats via the tail vein to simultaneously evaluate the functions of BCRP and P-gp in the BBB as well as the integrity of the BBB, respectively. At 40 min after treatment, the experimental rats were sacrificed under ether anesthesia. Blood samples were collected to prepare plasma and serum to measure the drug concentrations and biochemical parameters. The cerebral cortex and hippocampus were immediately harvested to assess the drug concentrations and the expression of the target proteins. The concentrations of prazosin and rhodamine 123 in the plasma and brain tissues were measured by the HPLC method [18, 24]. Fluorescein sodium was detected using the LC-MS method [18, 24]. The remaining brain tissues were used for Western blot analysis. The serum biochemical parameters, including AST, ALT ALP, TBA, TBIL, conjugated bilirubin and ammonia, were measured using commercial reagent kits, according to the instructions.
Cell culture and drug treatment
HCMEC/D3 cells were seeded in 24-well plates at a density of 2.0 × 105 cells/well and cultured in RPMI-1640 medium (RPMI-1640; Gibco-BRL, Eggenstein, Germany) supplemented with 10% FBS in 5% CO2 at 37 °C. The culture medium was refreshed daily. When the cells reached ~80% confluence, the cells were cultured in the medium containing NH4Cl (5 mM), UCB (10 μM or 25 μM), NH4Cl (5 mM) plus UCB (10 μM) and NH4Cl (5 mM) plus UCB (25 μM) for 72 h. The concentrations of NH4Cl and UCB were used based on previous reports [8, 17]. Then, the cultured cells were used to assess the expression and function of P-gp and BCRP.
Uptake of prazosin and rhodamine 123 by HCMEC/D3 cells
The uptake of prazosin and rhodamine 123 by HCMEC/D3 cells was measured to evaluate the function of BCRP and P-gp, according to previously described methods [18, 24]. In brief, the cultured cells were preincubated in 1 mL of Hanks’ balanced salt solution (HBSS) at 37 °C for 15 min. Then, the cells were incubated with 1 mL of HBSS containing prazosin (0.2 μM) or rhodamine 123 (0.2 μM) for another 120 min. The uptake reaction was terminated by washing three times with ice-cold HBSS. The intracellular concentrations of prazosin and rhodamine 123 were measured by HPLC methods [18, 24]. The protein concentrations were measured with a BCA protein assay kit (Beyotime Institute of Biotechnology, Shanghai, China).
Western blot analysis
The expression of the target proteins (BCRP, P-gp, ezrin, radixin, moesin and ERM protein complex) in HCMEC/D3 cells and brain tissues was measured using Western blot analysis. The brain tissues and cell samples were homogenized and lysed in RIPA lysis buffer. Plasma membrane proteins from the cerebral cortex and cells were prepared using a membrane protein extraction kit (Keygen Biotech Corp, Nanjing, China). Equal amounts of proteins were separated by 8% SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blocked in 5% skim milk for 2 h, followed by incubation with the indicated primary antibodies at 4 °C overnight. Then, the membranes were incubated with HRP-conjugated anti-rabbit G antibodies (1:5000 dilution) for 2 h. The immunoreactive bands were visualized on a gel imaging system (Tanon 5200 Multi, TanonTechnology Co., Ltd, Shanghai, China), and the intensities of the immunoreactive bands were quantified using ImageJ v.1.31 (available as freeware from http://rsbweb.nih.gov/ij/). The intensity values were normalized to the intensity of β-actin for total proteins and to the intensity of CD71 for membrane proteins [25,26,27]. The primary antibodies used were against P-gp (1:1000), BCRP(1:500), ezrin (1:1000), radixin (1:1000), moesin (1:1000), ERM complex protein (1:1000), β-actin (1:5000) and CD71 (1:1000).
Knockdown of ezrin, radixin and moesin with siRNA in HCMEC/D3 cells
To further confirm the contributions of ezrin, radixin and moesin to the altered membrane expression of the P-gp and BCRP proteins, ezrin, radixin and moesin were knocked down in HCMEC/D3 cells using Lipofectamine 3000 with the corresponding siRNA molecules (100 nM). siRNAs targeting ezrin (5′-UGAUUCUCGCGAUUAUUCUTT-3′), radixin (5′-CGACAAGUUAACACCUAAAUTT-3′) and moesin(5′-GCAGUACCAGGACACUAAATT-3′) were provided by GenePharma Co., Ltd. (Shanghai, China). After 6 h of transfection, the culture medium was replaced with fresh RPMI-1640 medium supplemented with 10% FBS. Following 18 h of incubation, the cells were incubated with the culture medium containing NH4Cl (5 mM) plus UCB (25 μM) for 72 h. Then, the protein expression of ezrin, radixin, moesin, P-gp and BCRP was evaluated using Western blot analysis. The results were compared with control cells (negative control siRNA) and cells without NH4Cl plus UCB treatment.
Data analysis
The results are presented as the mean ± standard deviation. Comparisons among multiple groups were performed by one-way analysis of variance followed by LSD (least significant difference) post hoc test. P < 0.05 indicated a significant difference.
Results
Alterations in the physiological and biochemical parameters of the BDL rats
The physiological and biochemical parameters of the rats were measured 14 days after BDL to confirm the occurrence of liver injury (Table 1). The results showed that compared with the SHAM rats, the BDL rats showed significant increases in the ALT, AST, ALP, TBIL, TBA and ammonia levels, which were accompanied by increased liver and spleen weight. The TBIL level in the BDL rats was increased by ~50-fold compared with that in the SHAM rats. These results demonstrated the successful establishment of BDL-induced liver failure in the rats.
Effects of BDL on the function and expression of BCRP and P-gp in the rat brain
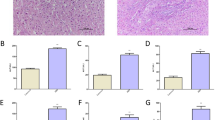
The distributions of prazosin, rhodamine 123 and fluorescein in the brain, presented as ratios of the concentrations in the brain to the concentrations in the plasma, were measured to assess the function of BCRP (Fig. 1a) and P-gp (Fig. 1b) as well as the integrity of the BBB (Fig. 1c). The results showed that BDL significantly increased the distribution of prazosin but decreased the distribution of rhodamine 123 without altering the distribution of fluorescein in the rat brain, indicating that BDL impaired BCRP function and enhanced P-gp function without affecting BBB integrity.
Ratios of prazosin (a), rhodamine 123 (b) and fluorescein (c) concentrations in the brain to those in the plasma. Total protein expression of BCRP (d) and P-gp (e) in the cerebral cortex and hippocampus; expression of the BCRP and P-gp proteins in the membrane from the cerebral cortex (f). The data are expressed as the mean ± SD (n = 6 for a, b, c and n = 8 for d, e, f). *P < 0.05, **P < 0.01 vs. the SHAM rats.
The protein expression of BCRP and P-gp was also measured using Western blotting. The results showed that consistent with the changes in the function of BCRP, BDL significantly downregulated the protein expression of BCRP in the brain (Fig. 1d). The protein expression of BCRP in the cortex and hippocampus of the BDL rats was decreased to 64% and 52% of that of the SHAM rats, respectively. In contrast to the alterations in the function of P-gp, the total protein expression of P-gp in the brain was clearly downregulated (Fig. 1e). The protein expression of P-gp in the cortex and hippocampus of the BDL rats was decreased to 62% and 70% of that of the SHAM rats, respectively. The expression of the BCRP and P-gp proteins on the plasma membrane in the cortex was further detected (Fig. 1f). Compared with the SHAM surgery, BDL downregulated the membrane expression of the BCRP protein but significantly upregulated the membrane expression of the P-gp protein by 2.3-fold.
Effects of NH4Cl, UCB and NH4Cl plus UCB on the function and expression of BCRP and P-gp in HCMEC/D3 cells
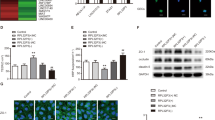
Our previous studies showed that increased UCB and ammonia levels in the serum were factors that impaired the expression and function of BCRP [8, 10]. Ammonia is also a factor that upregulates the expression and function of P-gp [17, 18]. The effects of NH4Cl, UCB and NH4Cl plus UCB on the function and expression of BCRP and P-gp were also determined using an in vitro HCMEC/D3 cell model. CCK-8 analysis demonstrated that these agents at the tested concentrations did not damage the cells (Fig. 2a). The results showed that a 72-h incubation with NH4Cl, UCB or NH4Cl plus UCB significantly increased prazosin uptake by the cells (Fig. 2b), suggesting an impairment of BCRP function. Consistent with the impaired function of BCRP, incubation with NH4Cl, UCB or NH4Cl plus UCB downregulated the protein expression of BCRP (Fig. 2d). No obvious cooperative effects of NH4Cl and UCB on the expression and function of BCRP were observed. This finding was consistent with previous reports [17, 18] that showed that ammonia induced the function and expression of P-gp (Fig. 2c, e). Incubation with UCB (25 μM) led to a decreasing trend in the function of P-gp, as evidenced by obvious increases in the uptake of rhodamine 123, but the total protein expression of P-gp was only minimally altered (Fig. 2c, e). Ammonia markedly attenuated the UCB-induced increased uptake of rhodamine 123 (Fig. 2c), and the uptake of rhodamine 123 was significantly lower than that of the control cells. Importantly, compared with the control, ammonia plus UCB obviously decreased the total protein expression of P-gp (Fig. 2e) by ~50%. The membrane expression of the BCRP and P-gp proteins was further detected. Consistent with the total protein expression of BCRP, ammonia, UCB and ammonia plus UCB significantly decreased the protein expression of BCRP (Fig. 2f). Ammonia slightly increased the membrane expression of the P-gp protein, but the difference was not significant. UCB alone did not affect the membrane expression of the P-gp protein. However, compared with the control, ammonia plus UCB markedly enhanced the membrane expression of the P-gp protein by 2.3-fold (Fig. 2g).
Cell activity (a), prazosin uptake (b), rhodamine 123 (Rho 123) uptake (c), total protein expression of BCRP (d) and total protein expression of P-gp (e), membrane expression of the BCRP protein (f) and membrane expression of the P-gp protein (g). The cells were incubated using the culture medium containing NH4Cl (5 mM), UCB (10 or 25 μM) or NH4Cl (5 mM) plus UCB (10 or 25 μM) for 72 h. The data are expressed as the mean ± SD (n = 6). *P < 0.05, **P < 0.01 vs. the control cells (CON). #P < 0.05, ##P < 0.01 vs. NH4Cl alone. $P < 0.05, $$P < 0.01 vs. UCB alone.
Alterations in the membrane localization of P-gp and BCRP was linked to the ERM proteins
The membrane localization of P-gp and BCRP [19,20,21,22,23] is linked to the ERM proteins. The expression of the ERM proteins ezrin, radixin and moesin in the brains of the BDL rats and cultured HCMEC/D3 cells was also measured. BDL significantly increased the total expression of the ERM complex proteins in the cortex. The total expression of the ERM complex proteins in the hippocampus showed an increasing trend, but the difference was not significant. Further study showed that the membrane expression of the ERM complex proteins in the cerebral cortex was also markedly increased by 2.2-fold compared with that in the SHAM rats (Fig. 3a). The total protein expression levels of ezrin, radixin and moesin and the levels of these proteins in the plasma membrane in the cerebral cortex were separately measured (Fig. 3b, c, d). The results showed that BDL significantly increased the total protein expression of ezrin but decreased the total protein expression of radixin in the cerebral cortex and hippocampus without affecting the total protein expression of moesin. This finding was consistent with the findings that BDL significantly upregulated the total protein expression of ezrin (increased by 2.6-fold compared with that in the SHAM rats) and downregulated the total protein expression of radixin (decreased to 39% compared with that in the SHAM rats) but did not alter the protein expression of moesin in the plasma membrane in the cerebral cortex.
Total expression of the ERM complex proteins in the cerebral cortex and hippocampus and plasma membrane expression of the ERM complex proteins (a). The ezrin protein, radixin protein, moesin protein in cerebral cortex (b) and hippocampus (c); expression of the ezrin protein, radixin protein, moesin protein (d) in the plasma membrane from cerebral cortex. The data are expressed as the mean ± SD (n = 8). *P < 0.05, **P < 0.01 vs. the SHAM rats.
The data from the HCMEC/D3 cells demonstrated that incubation with NH4Cl alone or NH4Cl plus UCB clearly increased the total expression of the ERM complex proteins (Fig. 4a), and NH4Cl plus UCB led to a stronger induction. Moreover, NH4Cl plus UCB markedly increased the membrane expression of the ERM complex proteins (Fig. 4b), and these increases did not occur in the cells treated with NH4Cl or UCB alone. NH4Cl, UCB or NH4Cl plus UCB significantly increased the total protein expression of ezrin, and NH4Cl plus UCB led to stronger increases (Fig. 4c). NH4Cl slightly increased the membrane expression of the ezrin protein, and this effect was further enhanced by the coadministration of UCB (Fig. 4d). NH4Cl, UCB and NH4Cl plus UCB significantly downregulated the expression of the total and membrane-associated radixin protein (Fig. 4e, f). NH4Cl, UCB or NH4Cl plus UCB only minimally affected the expression of the total and membrane-associated moesin protein (Fig. 4g, h). These results suggested that the increased membrane expression of the P-gp protein was associated with the increased membrane expression of the ezrin protein and that the decreased membrane expression of the BCRP protein was linked to the decreased membrane expression of the radixin protein.
Total expression (a) and membrane expression (b) of the ERM proteins, total expression (c) and membrane (d) expression of the ezrin protein, total expression (e) and membrane (f) expression of radixin protein, and total expression (g) and membrane (h) expression of the moesin protein in HCMEC/D3 cells treated with NH4Cl (5 mM), UCB (25 μM) and NH4Cl (5 mM) plus UCB (25 μM) for 72 h. The data are expressed as the mean ± SD (n = 6). *P < 0.05, **P < 0.01 vs. the control cells (CON). #P < 0.05, ##P < 0.01 vs. NH4Cl alone. $P < 0.05, $$P < 0.01 vs. UCB alone.
Roles of ezrin/radixin/moesin in the altered membrane expression of the BCRP and P-gp proteins by NH4Cl plus UCB in HCMEC/D3 cells
The roles of ERM in the altered membrane expression of the BCRP and P-gp proteins were investigated using siRNA. Western blot analysis showed that transfection with the indicated siRNA molecules significantly downregulated the membrane expression of ezrin, radixin and moesin (Fig. 5a, b, c), and these levels were decreased to ~9%, 36% and 52% of the levels observed in the control cells, respectively; these results demonstrated the successful silencing of these target genes. Silencing ezrin clearly reduced the membrane expression of the P-gp protein without affecting the membrane expression of the BCRP protein (Fig. 5a). Silencing radixin or moesin significantly downregulated the membrane expression of the BCRP protein but only minimally affected the membrane expression of the P-gp protein (Fig. 5b, c). The effect of NH4Cl (5 mM) plus UCB (25 μM) on the membrane expression of the BCRP and P-gp proteins was investigated in HCMEC/D3 cells after silencing ezrin, radixin or moesin. The results were consistent with our expectations that after silencing ezrin, radixin or moesin, incubation with NH4Cl (5 mM) plus UCB (25 μM) still exerted obvious inhibitory effects on the membrane expression of the BCRP protein (Fig. 5e), and knockdown of ezrin significantly downregulated the membrane expression of the P-gp protein and almost abolished the increased membrane expression of the P-gp protein induced by NH4Cl plus UCB (Fig. 5f).
Membrane expression of the ezrin, BCRP and P-gp proteins in HCMEC/D3 cells transfected with ezrin siRNA (a). Membrane expression of the radixin, BCRP and P-gp proteins in HCMEC/D3 cells transfected with radixin siRNA (b). Membrane expression of the moesin, BCRP and P-gp proteins in HCMEC/D3 cells transfected with moesin siRNA (c). Western blot of the effects of NH4Cl (5 mM) plus UCB (25 μM) on the membrane expression of the BCRP and P-gp proteins in HCMEC/D3 cells separately transfected with ezrin, radixin and moesin siRNA (d) and quantification of the Western blot results of the BCRP (e) and P-gp (f) levels. The data are expressed as the mean ± SD (n = 4). *P < 0.05, **P < 0.01 vs. the cells transfected with negative control siRNA (NC). #P < 0.05, ##P < 0.01, vssi-ezrin alone. $P < 0.05, $$P < 0.01, vssi-radixin alone. &P < 0.05, &&P < 0.01, vs si-moesin alone.
Discussion
Accumulating evidence has demonstrated that liver failure markedly alters the function and expression of P-gp, BCRP and MRP2 in the BBB [8,9,10,11], and these effects are dependent on the mechanism of liver failure and the species of ABC transporter [12]. P-gp and BCRP are often colocalized in the BBB and cooperatively regulate the transport of their substrates across the BBB [28,29,30]. The aim of this study was to simultaneously investigate the changes in the functions and expression of BCRP and P-gp in the brains of rats treated with BDL. The main findings were that BDL decreased BCRP function and increased P-gp function in the BBB of rats without affecting the integrity of the BBB. The decrease in BCRP function was consistent with the decreased total protein expression of BCRP. In contrast to the increased function of P-gp, the total protein expression of P-gp in the brain was significantly decreased. In general, the functions of BCRP and P-gp are linked to their membrane localization [31, 32], and the expression of BCRP and P-gp in the plasma membrane was measured. It was found that BDL significantly increased the membrane expression of the P-gp protein and decreased the membrane expression of the BCRP protein, suggesting that the alterations in their function were attributed to the altered membrane expression of these proteins.
Next, we focused on real mechanisms by which BDL oppositely regulates the expression and function of BCRP and P-gp in the BBB. Liver injury induced by BDL is often associated with hyperbilirubinemia and hyperammonemia. Significant increases in the levels of bilirubin and ammonia were also demonstrated in the serum of rats treated with BDL [33, 34]. The roles of ammonia and bilirubin in the altered expression and function of BCRP and P-gp were shown using an in vitro HCMEC/D3 cell model. The results were consistent with previous reports [8, 10] showing that bilirubin and ammonia impaired the expression and function of BCRP. The decreased function of BCRP was observed in parallel with the decreased total expression and membrane expression of the BCRP protein, suggesting that the impaired function and expression of BCRP in the BBB of rats treated with BDL were attributed to increased levels of bilirubin and ammonia. Ammonia induced the total protein expression of P-gp [17], which was consistent with the increased function of P-gp in vivo. However, the results did not explain the findings that BDL increased the function of P-gp in vivo, but the total protein expression of P-gp was decreased, suggesting the existence of other factors. The contributions of bilirubin, ammonia, and their combination to the expression and function of P-gp were further investigated. Ammonia increased the function and expression of P-gp, but bilirubin decreased the function of P-gp without affecting the total protein expression of P-gp. Importantly, the combination of bilirubin and ammonia markedly decreased the total protein expression of P-gp, although the function of P-gp was clearly increased. The data regarding the membrane expression of the proteins showed that the combination of bilirubin and ammonia markedly enhanced the membrane expression of the P-gp protein, which was consistent with the increased function of P-gp.
Next, we investigated factors that affect the membrane expression of the P-gp and BCRP proteins. ERM proteins, which are actin-binding linkers that connect the actin cytoskeleton to the plasma membrane, are often implicated in the membrane expression of proteins, including P-gp and BCRP [19,20,21,22,23]. Thus, whether alterations in the expression of BCRP and P-gp in the plasma membrane were also associated with ERM proteins was determined. The in vivo data showed that BDL significantly increased the expression of the ERM complex and ezrin proteins but decreased the expression of radixin in the plasma membrane without affecting the expression of moesin. In vitro, incubation with ammonia showed a trend toward increasing the membrane expression of the ezrin protein, and coadministration of bilirubin markedly increased the membrane expression of the ezrin protein by 3.2-fold of the expression observed in the control cells. In contrast, ammonia, bilirubin and their combination reduced the membrane expression of radixin without affecting the membrane expression of moesin. The in vitro data were consistent with the in vivo data. These results indicated that the increased membrane expression of P-gp was associated with the increased membrane expression of ezrin and that the decreased membrane expression of the BCRP protein was linked to the decreased membrane expression of the radixin protein. The roles of ezrin and radixin in the membrane expression of the P-gp and BCRP proteins were confirmed using targeted gene silencing. The results were consistent with our expectation that silencing ezrin significantly reduced the membrane expression of the P-gp protein (but not the BCRP protein) and almost abolished the increased membrane expression of the P-gp protein induced by NH4Cl plus UCB. In contrast, silencing radixin or moesin significantly downregulated the membrane expression of the BCRP protein (but not the P-gp protein). The combination of NH4Cl and UCB still showed obvious inhibitory effects on the membrane expression of the BCRP protein. BDL or NH4Cl plus UCB only minimally affected the membrane expression of the moesin protein. All these results suggested that decreased membrane expression of the BCRP protein resulted from impairment of the membrane expression of the radixin protein and that increased membrane expression of P-gp was due to the upregulated membrane expression of ezrin.
Other factors may be implicated in the opposite changes in the membrane expression of BCRP and P-gp. In general, P-gp and BCRP are localized to lipid rafts (cholesterol- and sphingolipid-enriched domains) of the plasma membrane [35, 36]. The functions of these proteins have also been demonstrated to be positively regulated by cholesterol [36, 37]. Increased cholesterol levels were also observed in the serum of the rats treated with BDL [38], which may, at least in part, explain the increased membrane expression of P-gp but not BCRP. Our previous studies also demonstrated that activation of the ROS-ERK1/2 pathway by ammonia enhanced the membrane expression of P-gp [18] but impaired the total protein expression of BCRP [10]. The present study also showed that decreases in the total protein expression BCRP were observed in parallel with downregulation of the membrane expression of the BCRP protein. All these factors may lead to opposite changes in the membrane expression of P-gp and BCRP, which needs further investigation.
The associations of membrane ERM protein expression with the membrane localization of the P-gp and BCRP proteins have been demonstrated in a series of studies, but the reports are often conflicting. Moreover, several investigations have indicated that the regulation of BCRP and P-gp by ERM proteins is organ- and cell-specific. In the small intestine, the expression profiles of radixin were consistent with those of P-gp [39]. Radixin−/− mice demonstrated reduced intestinal P-gp function [40]. In microvascular endothelial cells in the brain, an interaction between moesin colocalization and the P-gp protein was reported [41]. However, a report showed that P-gp coimmunoprecipitated with ezrin, not radixin or moesin, from total brain lysates [42], indicating an association between P-gp and ezrin in the brain, which was consistent with our data. In HCC827 cells, knockdown of ezrin or moesin significantly decreased the activities of P-gp and BCRP. In Caco-2 cells, ezrin or radixin knockdown only minimally affected BCRP function. In Caki-1 cells, radixin knockdown decreased BCRP function [21]. In HepG2 cells, ezrin knockdown decreased P-gp mRNA levels, and radixin knockdown decreased P-gp membrane expression [19]. Hoshietal reported that ezrin knockdown (46.6%) significantly decreased BCRP and slightly decreased P-gp and that radixin knockdown (50%) significantly decreased the membrane expression of P-gp and BCRP [23]; however, these results were different from our report. The discrepancies in the membrane expression of the P-gp protein occurred partially due to the efficiency of gene silencing or targeted cell line establishment. The true mechanisms still require further investigation.
In conclusion, BDL downregulated the function and the total and membrane expression of the BCRP protein but increased the function and the membrane expression of P-gp in the brains of rats. These results were attributed to the downregulated membrane expression of the radixin protein and the upregulation membrane expression of the ezrin protein, respectively, due to the integrated effects of hyperbilirubinemia and hyperammonemia.
Growing evidence has revealed that P-gp and BCRP in the BBB play vital roles in the physiological and pathological processes of the CNS and in drug treatment of CNS diseases. Hepatitis A, B and E are the predominant causes of liver failure in the developing world. Moreover, seizures are also often associated with liver failure [43]. Most antivirals (such as lamivudine, abacavir and zidovudine) [44,45,46] and some antiepileptics (such as lamotrigine and phenobarbital) [47, 48] have been demonstrated to be substrates of P-gp or BCRP. These drugs may be administered to patients suffering from liver failure, indicating that the altered expression and function of BCRP and P-gp in the BBB in the context of liver failure might affect the pharmacological/toxic effects of these drugs on the CNS; however, the data cannot be directly extrapolated to humans. ERM proteins participate in many functions, such as cell morphogenesis, endocytosis/exocytosis, adhesion and migration, through their versatile interactions with membrane transporters and transmembrane receptors/enzymes [49], indicating that in addition to altered P-gp and BCRP function and expression, alterations in the membrane expression of ERM proteins in brain tissues due to liver failure may be involved in the development of HE; this theory requires further investigation.
References
Wijdicks EF. Hepatic encephalopathy. N. Engl J Med 2016;315:1660–70.
Aldridge DR, Tranah EJ, Shawcross DL. Pathogenesis of hepatic encephalopathy: role of ammonia and systemic inflammation. J Clin Exp Hepatol. 2015;5:S7–S20.
Gil-Martins E, Barbosa DJ, Silva V, Remião F, Silva R. Dysfunction of ABC transporters at the blood-brain barrier: Role in neurological disorders. Pharmacol Ther. 2020;213:107554.
Sun H, Dai H, Shaik N, Elmquist WF. Drug efflux transporters in the CNS. Adv Drug Deliv Rev. 2003;55:83–105.
Ding Y, Wang R, Zhang J, Zhao A, Lu H, Li W, et al. Potential regulation mechanisms of P-gp in the blood-brain barrier in hypoxia. Curr Pharm Des. 2019;25:1041–51.
Cui Y, Lotz R, Rapp H, Klinder K, Himstedt A, Sauer A. Muscle to brain partitioning as measure of transporter-mediated efflux at the rat blood-brain barrier and its implementation into compound optimization in drug discovery. Pharmaceutics. 2019;11:595.
Liu L, Liu X. Contributions of drug transporters to blood-brain barriers. Adv Exp Med Biol. 2019;1141:407–66.
Xu P, Ling ZL, Zhang J, Li Y, Shu N, Zhong ZY, et al. Unconjugated bilirubin elevation impairs the function and expression of breast cancer resistance protein (BCRP) at the blood-brain barrier in bile duct-ligated rats. Acta Pharmacol Sin. 2016;37:1129–40.
Jin S, Wang XT, Liu L, Yao D, Liu C, Zhang M, et al. P-glycoprotein and multidrug resistance-associated protein 2 are oppositely altered in brain of rats with thioacetamide-induced acute liver failure. Liver Int. 2013;33:274–82.
Li Y, Zhang J, Xu P, Sun B, Zhong Z, Liu C, et al. Acute liver failure impairs function and expression of breast cancer-resistant protein (BCRP) at rat blood-brain barrier partly via ammonia-ROS-ERK1/2 activation. J Neurochem. 2016;138:282–94.
Liu L, Miao M, Chen Y, Wang Z, Sun B, Liu X, et al. The attenuated function and expression of P-glycoprotein at blood brain barrier of mice by acute liver failure and its clinical significance. Front Pharmacol. 2018;9:190.
Fan Y, Liu X. Alterations in expression and function of ABC family transporters at blood-brain barrier under liver failure and their clinical significances. Pharmaceutics. 2018;10:102.
Zwingmann C, Butterworth R. An update on the role of brain glutamine synthesis and its relation to cell-specific energy metabolism in the hyperammonemic brain: further studies using NMR spectroscopy. Neurochem Int. 2005;47:19–30.
Kerbert AJC, Jalan R. Recent advances in understanding and managing hepatic encephalopathy in chronic liver disease. F1000Res. 2020;9:F1000 Faculty Rev–312.
Gerth HU, Pohlen M, Pavenstädt H, Schmidt H. Extracorporeal liver support of liver failure. Z Gastroenterol. 2017;55:383–93.
Garcea G, Maddern GJ. Liver failure after major hepatic resection. J Hepatobiliary Pancreat Surg. 2009;16:145–55.
Zhang J, Zhang M, Sun B, Li Y, Xu P, Liu C, et al. Hyperammonemia enhances the function and expression of P-glycoprotein and Mrp2 at the blood-brain barrier through NF-κB. J Neurochem. 2014;131:791–802.
Zhou Y, Zhou J, Li P, Xie Q, Sun B, Li Y, et al. Increase in P-glycoprotein levels in the blood-brain barrier of partial portal vein ligation /chronic hyperammonemia rats is medicated by ammonia/reactive oxygen species/ERK1/2 activation: In vitro and in vivo studies. Eur J Pharmacol. 2019;846:119–27.
Kano T, Wada S, Morimoto K, Kato Y, Ogihara T. Effect of knockdown of ezrin, radixin, and moesin on P-glycoprotein function in HepG2 cells. J Pharm Sci. 2011;100:5308–14.
Yano K, Okabe C, Fujii K, Kato Y, Ogihara T. Regulation of breast cancer resistance protein and P-glycoprotein by ezrin, radixin and moesin in lung, intestinal and renal cancer cell lines. J Pharm Pharmacol. 2020;72:575–82.
Yano K, Otsuka K, Kato Y, Kawabata H, Ohmori S, Arakawa H, et al. Different regulation of P-glycoprotein function between Caco-2 and Caki-1 cells by ezrin, radixin and moesin proteins. J Pharm Pharmacol. 2016;68:361–7.
Pokharel D, Padula MP, Lu JF, Jaiswal R, Djordjevic SP, Bebawy M. The role of CD44 and ERM proteins in expression and functionality of P-glycoprotein in breast cancer cells. Molecules. 2016;21:290.
Hoshi Y, Uchida Y, Kuroda T, Tachikawa M, Couraud PO, Suzuki T, et al. Distinct roles of ezrin, radixin and moesin in maintaining the plasma membrane localizations and functions of human blood-brain barrier transporters. J Cereb Blood Flow Metab. 2020;40:1533–45.
Qin YY, Xu P, Wu T, Qian CQ, Fan YL, Gen DH, et al. Bile duct ligation enhances AZT CNS toxicity partly by impairing the expression and function of BCRP in rat brain. Acta Pharmacol Sin. 2020;41:181–91.
Shao M, Peng ZX, Shi CY, Tang R, Manzo LM, Liu Y. A convenient method for hTfR1 inclusion body purification. Prep Biochem Biotechnol. 2015;45:743–53.
Wilner SE, Wengerter B, Maier K, de Lourdes BorbaMagalhães M, Del Amo DS, Pai S, et al. An RNA alternative to human transferrin: a new tool for targeting human cells. Mol Ther Nucleic Acids. 2012;1:e21.
Li S, Wang X, Ma QH, Yang WL, Zhang XG, Dawe GS. Amyloid precursor protein modulates Nav1.6 sodium channel currents through a Go-coupled JNK pathway. Sci Rep. 2016;6:39320.
Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25.
Begley D. ABC transporters and the blood-brain barrier. Curr Pharm Des. 2004;10:1295–312.
Scherrmann J. Expression and function of multidrug resistance transporters at the blood-brain barriers. Expert Opin Drug Metab Toxicol. 2005;1:233–46.
Liu XD. ABC family transporters. AdvExp Med Biol. 2019;1141:13–100.
Amawi H, Sim HM, Tiwari AK, Ambudkar SV, Shukla S. ABC transporter-mediated multidrug-resistant cancer. Adv Exp Med Biol. 2019;1141:549–80.
Huang HC, Chang CC, Wang SS, Chan CY, LeeFT, Chuang CL, et al. Pravastatin for thioacetamide-induced hepatic failure and encephalopathy. Eur J Clin Invest. 2012;42:139–45.
Mendes NF, Mariotti FFN, de Andrade JS, de Barros Viana M, Céspedes IC, Nagaoka MR, et al. Lactulose decreases neuronal activation and attenuates motor behavioral deficits in hyperammonemic rats. Metab Brain Dis. 2017;32:2073–83.
Radeva G, Perabo J, Sharom FJ. P-glycoprotein is localized in intermediate-density membrane microdomains distinct from classical lipid rafts and caveolar domains. FEBS J. 2005;272:4924–37.
Storch CH, EhehaltR, Haefeli WE, Weiss J. Localization of the human breast cancer resistance protein (BCRP/ABCG2) in lipid rafts/caveolae and modulation of its activity by cholesterol in vitro. J Pharmacol Exp Ther. 2007;323:257–64.
Gayet L, Dayan G, Barakat S, Labialle S, Michaud M, Cogne S, et al. Control of P-glycoprotein activity by membrane cholesterol amounts and their relation to multidrug resistance in human CEM leukemia cells. Biochemistry. 2005;44:4499–509.
Tanaka S, Kinowaki M, Maeda Y, Nagatomo J, Kai MH, Kondo KH, et al. Speciesdifference incholesterol7alpha-hydroxylase expression of rabbit and rat liver microsomes after bile duct ligation. J Surg Res. 2004;119:36–40.
Kobori T, Harada S, Nakamoto K, Tokuyama S. Radixin influences the changes in the small intestinal P-glycoprotein by etoposide treatment. Biol Pharm Bull. 2013;36:1822–8.
Yano K, Tomono T, Sakai R, Kano T, Morimoto K, Kato Y, et al. Contribution of radixin to P-glycoprotein expression and transport activity in mouse small intestine in vivo. J Pharm Sci. 2013;102:2875–81.
Kobori T, Fujiwara S, Miyagi K, Harada S, Nakamoto K, Nakagawa T, et al. Involvement of moesin in the development of morphine analgesic tolerance through P-glycoprotein at the blood-brain barrier. Drug Metab Pharmacokinet. 2014;29:482–9.
Zhang Y, Dong J, Zhu X, Wang W, Yang Q. The effect of sphingomyelin synthase 2 (SMS2) deficiency on the expression of drug transporters in mouse brain. Biochem Pharmacol. 2011;82:287–94.
Shawcross DL, Wendon A. The neurological manifestations of acute liver failure. Neurochem Inter. 2012;60:662–71.
De Souza J, Benet LZ, Huang Y, Storpirtis S. Comparison of bidirectional lamivudine and zidovudine transport using MDCK, MDCK-MDR1, and Caco-2 cell monolayers. J Pharm Sci. 2009;98:4413–9.
Shaik N, Giri N, Pan G, Elmquist WF. P-glycoprotein-mediated active efflux of the anti-HIV1 nucleoside abacavir limits cellular accumulation and brain distribution. Drug Metab Dispos. 2007;35:2076–85.
Pan G, Giri N, Elmquist WF. Abcg2/Bcrp1 mediates the polarized transport of antiretroviral nucleosides abacavir and zidovudine. Drug Metab Dispos. 2007;35:1165–73.
Römermann K, Helmer R, Löscher W. The antiepileptic druglamotrigineis a substrate of mouse and human breast cancer resistance protein (ABCG2). Neuropharmacology. 2015;93:7–14.
Yang ZH, Liu XD. P-glycoprotein-mediated efflux of phenobarbital at the blood-brain barrier evidence from transport experiments in vitro. Epilepsy Res. 2008;78:40–9.
Clucas J, Valderrama F. ERM proteins in cancer progression. J Cell Sci. 2014;127:267–75.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Nos. 81872930, 82073922, 81673505), and the “Double First-Class” University Project (CPU2018GY22).
Author information
Authors and Affiliations
Contributions
TW, YYQ, XDL, and LL designed the experiments. TW and XDL analyzed the data. TW, XDL and LL wrote the manuscript. TW, YS, WMK, MMJ, HYY, XKZ, CD, ML conducted experiments. TW, YS, XDL and LL reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Wu, T., Sheng, Y., Qin, Yy. et al. Bile duct ligation causes opposite impacts on the expression and function of BCRP and P-gp in rat brain partly via affecting membrane expression of ezrin/radixin/moesin proteins. Acta Pharmacol Sin 42, 1942–1950 (2021). https://doi.org/10.1038/s41401-020-00602-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-020-00602-3