Abstract
Larger thalamic volume has been found in children with obsessive-compulsive disorder (OCD) and children with clinical-level symptoms within the general population. Particular thalamic subregions may drive these differences. The ENIGMA-OCD working group conducted mega- and meta-analyses to study thalamic subregional volume in OCD across the lifespan. Structural T1-weighted brain magnetic resonance imaging (MRI) scans from 2649 OCD patients and 2774 healthy controls across 29 sites (50 datasets) were processed using the FreeSurfer built-in ThalamicNuclei pipeline to extract five thalamic subregions. Volume measures were harmonized for site effects using ComBat before running separate multiple linear regression models for children, adolescents, and adults to estimate volumetric group differences. All analyses were pre-registered (https://osf.io/73dvy) and adjusted for age, sex and intracranial volume. Unmedicated pediatric OCD patients (<12 years) had larger lateral (d = 0.46), pulvinar (d = 0.33), ventral (d = 0.35) and whole thalamus (d = 0.40) volumes at unadjusted p-values <0.05. Adolescent patients showed no volumetric differences. Adult OCD patients compared with controls had smaller volumes across all subregions (anterior, lateral, pulvinar, medial, and ventral) and smaller whole thalamic volume (d = −0.15 to −0.07) after multiple comparisons correction, mostly driven by medicated patients and associated with symptom severity. The anterior thalamus was also significantly smaller in patients after adjusting for thalamus size. Our results suggest that OCD-related thalamic volume differences are global and not driven by particular subregions and that the direction of effects are driven by both age and medication status.
Similar content being viewed by others
Introduction
Obsessive-compulsive disorder (OCD) is a debilitating mental disorder with a lifetime prevalence of 1.3% that often starts during childhood and follows a chronic course [1]. Structural and functional differences in cortico-striato-thalamo-cortical (CSTC) circuits have been linked to commonly observed affective and cognitive deficits, including fear conditioning and extinction, planning ability, response inhibition, habitual behaviour, and emotion regulation [2]. The thalamus lies at the core of these functionally segregated CSTC circuits. Previous work from the ENIGMA-OCD Working Group revealed that the thalamus is larger in unmedicated pediatric OCD patients compared with controls [3] and shows more leftward asymmetry [4]. The former finding was corroborated in the population-based Generation R study, showing that school-aged children with symptoms above a clinical cut-off had a larger thalamus than symptom-free children [5].
Thalamus structure is not uniform and consists of several nuclei with distinct connectivity and functions [6]. The anterior nucleus connects fronto-limbic regions including the medial orbitofrontal and ventromedial prefrontal cortex [7]. The mediodorsal nucleus is involved in both emotional and cognitive processing, including reward devaluation, fear extinction, working memory, behavioural flexibility, and goal-directed behaviour [8, 9]. The pulvinar nucleus functions as an association nucleus involved in attributing salience to visual stimuli and modulating attention and behaviour accordingly [9, 10]. The ventral thalamus includes the ventral anterior and ventral lateral nucleus, which are involved in motor control [9]. Functional deficits in all of the aforementioned domains and their associated circuits have been implicated in the neurobiological model of OCD [2]. Therefore, larger thalamus volume in OCD may reflect structural or functional changes within these circuits that are expressed in enlarged volume of specific nuclei.
Few studies have investigated OCD-related morphological differences of the thalamus at the subregional level. Shape analysis has found increased surface area of the anterior and pulvinar nuclei in adult OCD patients compared with controls [11]. Within school-aged children from the Generation R study, all thalamic nuclei except the pulvinar trended towards larger volumes in the clinical symptom group compared with children without symptoms [12]. The ventral nucleus group showed the largest difference, though this was no longer significant after correction for multiple comparisons [12]. Another recent study found that adult patients compared with controls had lower volumes of the left posterior thalamus, which includes the pulvinar and geniculate nuclei, and smaller posterior thalamus volume was also associated with a later age of onset of OCD [13]. A limitation of previous literature is the focus on a specific age group, limiting the ability to explore differences across the lifespan. The datasets of ENIGMA-OCD allow comparison across a larger age range, including children, adolescents and adults.
We performed mega- and meta-analyses comparing thalamic nuclei volume between OCD patients and healthy controls across 29 sites worldwide. We used the automated segmentation algorithm implemented in FreeSurfer 7.1.1 that uses a histology-based probabilistic atlas created by Iglesias and colleagues [14]. We also examined the effects of medication status, age, sex, age of disease onset, and symptom severity. Based on the literature, we expected that pediatric OCD patients would show larger nuclei volumes compared with healthy controls, mostly driven by larger volume of the anterior and ventral thalamus. Furthermore, adult OCD patients were expected to have lower pulvinar volumes compared with healthy controls [13]. Based on our previous analyses on subcortical and cortical morphology, we expected that volume enlargement in children would be driven by unmedicated patients and lower volume in adults by medicated patients [3, 15]. We also expected that adult OCD patients with a childhood onset would show larger nuclei volumes than patients with adult-onset OCD.
Materials and methods
Samples
The ENIGMA-OCD working group is a collaboration of 29 international research institutes that provided in total 50 samples of structural brain imaging and clinical data from OCD patients and control participants without psychopathology. These samples partly overlap with the previous cortical and subcortical mega-analyses performed by this group [3, 15]. We divided the participants into three age groups: children under 12 years, adolescents aged 12–17 years, and adults aged 18 years and older. After excluding 45 participants based on poor segmentations, the dataset included 5423 participants, among which 2678 OCD patients (96 children, 317 adolescents, 2265 adults) and 2746 healthy controls (90 children, 254 adolescents, 2402 adults). The institutional review boards of each participating site permitted the analysis of measures extracted from coded, de-identified individual participant data.
Image acquisition and processing
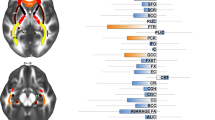
T1-weighted structural brain magnetic resonance imaging (MRI) scans of the participants were acquired at each site and processed using the latest stable release of FreeSurfer (version 7.1.1) for segmentation of the whole thalamus. Next, the pipeline of Iglesias and colleagues [14] was used to segment the thalamus into 25 nuclei, which we grouped into five different subregions per hemisphere: anterior, lateral, ventral, intralaminar/medial and pulvinar (see Fig. 1 for an overview of the subdivision and Fig. S1 for an in vivo example). A containerized version (Singularity) of the pipeline was created to facilitate cross-platform compatibility at each site (http://datasets.datalad.org/?dir=/shub/chrisvriend/ENIGMA_subthal) and allow standardized quality inspection of the segmentation by means of custom-made Python and shell scripts. All segmentations were visually inspected and investigated for statistical outliers. Full details of the image exclusion criteria and quality assessments are described in Supplementary Section S1.
Statistical analysis
We conducted both mega- and meta-analyses. Statistical analyses were conducted in accordance with statistical protocols of the ENIGMA Consortium (http://enigma.ini.usc.edu/). We pre-registered our analysis plan and hypotheses prior to conducting any analyses (https://osf.io/73dvy). Mega-analyses were performed by pooling the extracted volume measures for each participant across all sites. The data were harmonized to adjust for possible cross-site batch effects using a modified version of the ComBat function [16]. Multiple linear regression models were run on the harmonized data. We conducted separate analyses for children, adolescents and adults. The bilateral mean volumes of the five subregions served as the main outcome measures. Diagnosis was used as a binary predictor in all analyses. All models were adjusted for age, sex, and total intracranial volume (ICV). We additionally investigated the interaction of age, age-squared, sex or sex-by-age. Details of the meta-analysis are described in Supplementary Section S2.
We computed Cohen’s d (d) effect sizes using the t-statistic of the main predictor variable in the regression models. Medication effects were investigated by conducting stratified analyses comparing medicated versus unmedicated OCD patients with the healthy control group. Similarly, we conducted stratified analyses for the adult-onset and child-onset (onset before age 18 years) groups to study the influence of time of onset (age at diagnosis). We also investigated the continuous association of subregional volume with illness severity, expressed in terms of the total severity score of the adult and child versions of the Yale-Brown Obsessive Compulsive Scale (Y-BOCS) [17, 18]. In post hoc (i.e. not pre-registered) analyses, lateralization of volume differences was assessed in children and adults by computing an asymmetry index ([L – R]/([L + R]/2)) for each subregion [4]. We further explored subregional volume differences relative to the whole thalamus by post hoc adjustment for whole thalamus volume. Since subregions and whole thalamus are highly correlated, we evaluated variance inflation factors to rule out any multicollinearity in the models. Here, we also statistically compared effect sizes between subregions using Z-tests. The main analyses were corrected for multiple comparisons by applying a Benjamini–Hochberg false discovery rate correction (q = 0.05) across the five subregions tested. We also report uncorrected results throughout the manuscript.
Results
Table 1 provides an overview of the demographic and clinical characteristics of the pooled samples for each age group. The demographic and clinical characteristics of the separate samples are displayed in Table S1 in the data supplement.
Mega-analysis
Thalamic subregional volume differences between OCD patients and controls
Children (<12 years)
Consistent with previous findings, larger volume between children with OCD (n = 96) compared with controls (n = 90) of the whole thalamus (d = 0.32 [0.03, 0.61], p = 0.03). Larger volume was observed in children with OCD compared with healthy controls in the lateral subregion (d = 0.37 [0.08, 0.66], p = 0.01, pFDR = 0.08), but the difference did not survive correction for multiple comparisons. The other subregions trended towards increased volume in OCD patients compared with controls (d = 0.16 [−0.133, 0.445] to 0.28 [−0.008, 0.576], unadjusted p = 0.06 to 0.30, pFDR = 0.10 to 0.30) (see Fig. 2 and Table 2). We found no significant differences in subregional volume after adjusting for whole thalamus volume (see Table S2).
Adolescents (12–17 years)
There were no significant volume differences of the thalamus as a whole or subregions between adolescent OCD patients (n = 317) and healthy controls (n = 354) (see Fig. 2 and Table 2). There was also no group differences in relative thalamus volume (Table S3).
Adults (18–69 years)
Whole thalamus volume was smaller in adult OCD patients (n = 2265) compared with controls (n = 2402) (d = −0.11 [−0.17, −0.05], p = 0.001). All subregions had smaller volume in adult OCD patients compared with healthy controls (d = −0.08 [−0.133, −0.017] to −0.15 [−0.21, −0.09], pFDR = <0.001 to 0.011, Fig. 2 and Table 2) and in the whole thalamus. In addition, anterior volume relative to whole thalamus volume was significantly smaller in adults patients (d = −0.10 [−0.16, −0.05], pFDR = 0.003, Table S4). We ruled out multicollinearity of these models by inspecting variance inflation factors, which all remained below 2. Formal comparisons of effect sizes between the anterior thalamus and other subregions revealed statistically significant or nearly significant differences with the pulvinar (Z = −2.45, p = 0.0144), ventral (Z = −3.75, p < 0.001), lateral (Z = −1.92, p = 0.0546) and medial (Z = −1.94, p = 0.0658) thalamus.
Influence of medication status
Figure 3 visualizes the volumetric differences between medicated patients, unmedicated patients and healthy controls in relation to age and sex.
Children
Unmedicated children with OCD (n = 75) compared with healthy controls (n = 88) had a larger lateral volume (d = 0.46 [0.15, 0.77], pFDR = 0.02). At an unadjusted significance threshold, patients had a larger ventral subregion (d = 0.35 [0.04, 0.66], p = 0.03, pFDR = 0.07) and pulvinar (d = 0.33 [0.02, 0.64], p = 0.03, pFDR = 0.07) (see Table S5). We found no significant volume differences between medicated children with OCD and healthy controls or between medicated and unmedicated children with OCD (see Tables S6 and S7).
Adolescents
We found no significant differences between medicated adolescents with OCD (n = 186), unmedicated adolescent OCD patients (n = 128) and healthy controls (n = 254) (see Tables S8–S10).
Adults
Medicated adults with OCD (n = 1091) compared with healthy controls (n = 2402) exhibited a significantly smaller volume of all thalamic subregions (d = −0.09 [−0.16, −0.01] to −0.17 [−0.24, −0.10], pFDR = <0.001 to 0.02) (Table S11), but there were no significant differences between unmedicated adult OCD patients (n = 1000) and healthy controls (n = 2402 (Table S12) or between medicated and unmedicated patients (Table S13).
Influence of age and sex on OCD-related differences in thalamic nuclei volume
Results of the interaction models are displayed in Tables S14–S25. In children, we found an age-by-diagnosis interaction for the medial (Cohen’s d = 0.30 [0.01, 0.60], p = 0.046, pFDR = 0.076), pulvinar (d = 0.31 [0.02, 0.60], p = 0.041, pFDR = 0.076), ventral (d = 0.36 [0.07, 0.65], p = 0.018, pFDR = 0.076) nuclei and for the whole thalamus (d = 0.36 [0.07, 0.65], p = 0.017). This indicates larger volume with age in patients versus lower volume with age in controls (see Table S15). We also found an age-by-sex-by-diagnosis interaction in the anterior (d = −0.32 [−0.61, −0.03], pFDR = 0.045), medial (d = −0.40 [−0.70, −0.11], pFDR = 0.026), pulvinar (d = −0.39 [−0.69, −0.10], pFDR = 0.026), ventral (d = −0.34 [−0.63, −0.05], pFDR = 0.045) subregions and for the whole thalamus (d = −0.42 [−0.71, −0.13], p = 0.006) (see Table S17), indicative of larger volume in male pediatric OCD patients with age, but lower volume with age in female OCD patients and healthy controls. We found no such interactions in adolescents (Table S18–S21).
In adults, we found a sex-by-diagnosis interaction in the anterior subregion (d = −0.07 [−0.12, −0.01]; p = 0.03, pFDR = 0.13) (see Table S22), indicating stronger volume differences between OCD patients and healthy controls among females. We found no other interactions (see Tables S23–S25).
Influence of time of disease onset on thalamic subregional volume in adults
Compared with healthy controls (n = 2402), adult-onset OCD patients (n = 1000) had a smaller medial (d = −0.10 [−0.17, −0.02], pFDR = 0.026) and anterior (d = −0.14 [−0.21, −0.07], pFDR = 0.001) thalamic volume. The ventral and lateral subregions were smaller at unadjusted p-value < 0.05 (see Table S26). There were no differences between adult patients with a child-onset (n = 850) and controls (see Table S27) or between child-onset and adult-onset patients (see Table S28).
Association between illness severity and thalamic subregional volume
Medication use was associated with higher YBOCS scores (t = 2.23, p = 0.026) and the presence of comorbid depressive disorders (t = 3.13, p = 0.002) but not comorbid anxiety disorders (t = 0.59, p = 0.56). We therefore additionally adjusted the severity analyses for medication use and comorbid depressive disorders. There was no relationship between symptom severity and subregional volume in pediatric (n = 83) and adolescent OCD patients (n = 301) (see Table S29 and S30). Conversely, symptom severity in adult OCD patients (n = 2106) was negatively associated with volume in all subregions (r = −0.04 and −0.06) and whole thalamus volume (r = −0.07) (see Table S31).
Volume asymmetry group differences between patients and controls
We found no significant thalamic subregional volume asymmetry in child, adolescent and adult OCD patients compared with controls (see Tables S32–S34).
Meta-analysis
Methods and results of the meta-analysis are displayed in Supplementary Section S1 and Table S35 and Fig. S1. The results of the meta-analysis corroborate the mega-analysis results.
Discussion
We performed a mega- and meta-analysis investigating differences in thalamic subregion volume between OCD patients and healthy controls across the lifespan. While unmedicated pediatric patients had significantly larger lateral volumes and larger pulvinar, ventral and whole thalamus volumes at uncorrected significance thresholds compared with controls, adult OCD patients had smaller volumes across all subregions, which was mostly driven by medicated and adult-onset patients. No volume differences were found in adolescent OCD patients. Furthermore, most subregional group differences did not persist after adjusting for whole thalamus volume. Together, this provides new insight that OCD-related thalamic volume differences are global and not driven by particular subregions and that the direction of effects are explained by both age and medication status.
The current study further elucidates the strongly age-dependent nature of the link between OCD and thalamic morphology. In line with our prior work, we found the whole thalamus was larger in the pediatric patient group [3]. However, a crucial difference is that we split the pediatric group into children (<12 years) and adolescents (12–17 years). This revealed that OCD-related thalamic volume differences appear during childhood and already attenuate during adolescence. Complementary to the pediatric findings and in contrast with our previous work, we found significantly smaller volumes across all subregions in adult OCD patients compared with controls. This novel finding relative to previous work is likely explained by increased power from the larger sample size and use of the ComBat algorithm to adjust for batch effects [16], as well as the more sensitive thalamic segmentation method [14]. Taken together, we robustly show that thalamic volume differences, regardless of subregions, follow an opposite trajectory from larger to smaller volumes across age in patients compared with controls. This is consistent with the significant interactions with age and age-by-sex we found in the pediatric sample. However, we are cautious to interpret the pediatric interaction analyses due to the low sample size and skewed age distribution in this group. Conversely, the sufficiently powered adult sample size did not show a significant interaction with age or age-by-sex. This could indicate that differences in volume trajectories across age between patients and controls that appear in the plot are challenging to model with formal testing. However, we did find a sex-by-diagnosis interaction in adults for the anterior subregion (see Table S3), indicating that OCD-related volume differences are on average stronger in females than males. We should note that our findings in adults are partly consistent with recent work from Jurng et al. in a sample that was also included in the current analysis [13]. While the current study found lower volumes of all subregions in adult OCD patients, Jurng et al. found significant differences only in the posterior thalamus region that includes the pulvinar [13]. This difference is most likely explained by differences in sample size and the fact that only unmedicated patients were included in the Jurng et al. sample.
Given that specific thalamic subregions play a key role in OCD-relevant circuitry [2], we hypothesized morphometric differences in these subregions in OCD. Thalamic subregions have been more intensively studied in relation to schizophrenia and have repeatedly shown subregional volume differences [19], including recently by using the same Iglesias et al. segmentation tool [20, 21]. Participants at high risk of developing schizophrenia with auditory hallucinations had a smaller volume of the medial geniculate nucleus and a steeper volume decrease over time compared with symptom-free controls, accompanied by functional connectivity differences in related circuits [21]. This suggests that subregional volume changes might be related to specific symptoms. OCD-related symptom dimensions have also been related to distinct structural changes [22]. In the current study, we only had data on global symptom severity and not specific symptom dimensions. Given the heterogeneity of the OCD phenotype and the many circuits involved, this could explain why the shrinkage of the thalamus was distributed across all subregions.
Conversely, thalamus volume was larger rather than smaller in the child sample, suggesting another process may be at play. For instance, the larger overall thalamus volume could reflect broad neurodevelopmental differences between patients and controls that predispose to developing OCD. During early childhood and adolescence, thalamo-cortical circuits undergo a process of myelination, synaptic remodelling and pruning that give rise to a mature thalamus [23]. Recent work showed that compulsivity and impulsivity are related to reduced myelin-related growth in cortico-striatal regions in adolescents [24]. Although disagreement exists on the trajectory of normative thalamic development, relative thalamus size has been described to increase until 4 years of age [25], followed by either a steady decrease [26] or stabilization [25] during childhood and adolescence. The larger volumes observed in our pediatric OCD patients aged 6–12 years may therefore reflect altered neurodevelopment. The association was strongest in unmedicated children, indicating that medication normalizes thalamic volume. This is consistent with the report by Gilbert et al. [27], that revealed enlargement of the thalamus in pediatric patients that normalized after 12-week-treatment with paroxetine. Since neurodevelopment is governed by both genetic [28] and environmental influences [29], longitudinal studies integrating these aspects can offer insight into the altered neurodevelopment in OCD.
Thalamic volume declines with healthy ageing [30] and shows enhanced decline in various brain disorders [31, 32]. Likewise, OCD patients may exhibit a more rapid decline in thalamic volume through various mechanisms. Glutamatergic neurons fulfil an important role within the CSTC circuits. Several studies have found alterations in glutamatergic systems in OCD, indicating possible glutamatergic dysregulation [33, 34]. Excitotoxic effects of glutamate have been proposed as a mechanism for local structural loss in schizophrenia [35]. Alternatively, the presence of auto-antibodies in conjunction with increased excitatory metabolites in adult OCD suggest activity of autoimmune processes [34].
Our results suggest that medication status largely drives volumetric differences in adult OCD. Selective serotonin reuptake inhibitors (SSRIs) have been reported to induce both neurogenesis and neuronal elimination in the hippocampus [36, 37]. In vitro studies have linked antidepressant exposure to non-neuronal and neuronal apoptosis or death [38, 39], providing a possible mechanism for volume decline of brain structures. However, to our knowledge, such effects have not been described in the thalamus. In another study, a single dose of SSRIs caused widespread decreases in subcortical and cortical connectivity as well as increased thalamic connectivity [40]. Perhaps functional alterations induce structural remodelling of the thalamus that result in volumetric changes. However, causal effects cannot be inferred due to the cross-sectional methodology and lack of detailed information on medication status.
Only the anterior subregion was significantly smaller relative to overall thalamic volume in adult OCD patients, suggesting that this difference is superimposed on the overall thalamic difference. Formal comparison of effect sizes between the anterior region and other subregions using Z-tests showed (near) significant differences across regions. This suggests that on top of a general case-control difference in thalamus volume, the anterior subregion shows the most pronounced contribution to smaller volume in adult OCD. As the variance inflation factors were in the range 1.65–1.66 for the different subregions, there was no evidence of multicollinearity that may have influenced these findings. The ventral anterior nucleus connects to fronto-limbic regions including the medial orbitofrontal and ventromedial prefrontal cortex [7]. Lower relative volume of the anterior nucleus may reflect fronto-limbic disruption, which has been linked to the dysregulated fear responses in OCD, characterized by excessive and poorly controlled fear responses to stimuli [2].
Strengths of our study include the large sample size, the use of a harmonized and containerized processing protocol and quality inspection procedure, as well as adjustment for batch effects that arise from multi-site data. Furthermore, separation into three age groups allowed us to identify stage-specific volume differences. Nevertheless, the cross-sectional nature of the study complicates identifying developmental processes and medication effects that seem to underlie the observed volume effects. Furthermore, despite adjustment for batch effects, we are not able to overcome all effects that arise from scanner and site differences. Since all sites included both patient and control groups, large effects of site differences are, however, not expected. Despite the large sample size, adults form the majority of participants and pediatric participants are relatively underrepresented. It is important to emphasize that because of this not all findings in the pediatric sample survived multiple comparisons. Fortunately, the ENIGMA-OCD Working Group continues to grow, and future analyses will enable us to balance out the age distribution in favour of younger participants. We should also acknowledge that the internal borders of the thalamus on T1 images are difficult to discern with visual checks and could not be used to visually verify the anatomical correctness of the segmentations. Despite this, the pipeline’s validity has been thoroughly evaluated with histological comparison, across different field strengths (1.5T and 3T) and has successfully detected subregional differences across different disorders and age groups [12, 14, 20, 21].
Despite these limitations, our results provide the first robust findings on OCD-related volume differences in the thalamus across different developmental stages. In contrast with our hypotheses, we observed mostly global thalamic rather than region-specific volume differences, suggesting that the relationship with OCD is not driven by changes of a particular subregion but overall thalamus volume. Future endeavours should focus on disentangling longitudinal age-related and medication-related effects to determine what drives these global differences. Furthermore, multimodal approaches including resting-state functional MRI and diffusion-weighted imaging could bolster our understanding of thalamic connectivity and its involvement in OCD, preferably at higher field strengths to attain optimal contrast for high-quality images.
References
Fawcett EJ, Power H, Fawcett JM. Women are at greater risk of OCD than men: a meta-analytic review of OCD prevalence worldwide. J Clin Psychiatry. 2020;81.
Shephard E, Stern ER, van den Heuvel OA, Costa DLC, Batistuzzo MC, Godoy PBG, et al. Toward a neurocircuit-based taxonomy to guide treatment of obsessive–compulsive disorder. Mol Psychiatry. 2021.
Boedhoe PS, Schmaal L, Abe Y, Ameis SH, Arnold PD, Batistuzzo MC, et al. Distinct subcortical volume alterations in pediatric and adult OCD: a worldwide meta- and mega-analysis. Am J Psychiatry. 2017;174:60–9.
Kong XZ, Boedhoe PSW, Abe Y, Alonso P, Ameis SH, Arnold PD, et al. Mapping cortical and subcortical asymmetry in obsessive-compulsive disorder: findings from the ENIGMA consortium. Biol Psychiatry. 2020;87:1022–34.
Weeland CJ, White T, Vriend C, Muetzel RL, Starreveld J, Hillegers MHJ, et al. Brain morphology associated with obsessive-compulsive symptoms in 2,551 children from the general population. J Am Acad Child Adolesc Psychiatry. 2021;60:470–8.
Behrens TEJ, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CAM, Boulby PA, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6:750–7.
Grodd W, Kumar VJ, Schüz A, Lindig T, Scheffler K. The anterior and medial thalamic nuclei and the human limbic system: tracing the structural connectivity using diffusion-weighted imaging. Sci Rep. 2020;10:10957.
Benarroch EE. Pulvinar: associative role in cortical function and clinical correlations. Neurology. 2015;84:738–47.
Nieuwenhuys R, Voogd J, van Huijzen C. Diencephalon: dorsal thalamus. In: The Human Central Nervous System. 2008. p. 253–79.
Mitchell AS, Browning PGF, Baxter MG. Neurotoxic lesions of the medial mediodorsal nucleus of the thalamus disrupt reinforcer devaluation effects in rhesus monkeys. J Neurosci. 2007;27:11289–95.
Shaw P, Sharp W, Sudre G, Wharton A, Greenstein D, Raznahan A, et al. Subcortical and cortical morphological anomalies as an endophenotype in obsessive-compulsive disorder. Mol Psychiatry. 2015;20:224–31.
Weeland CJ, Vriend C, van der Werf Y, Huyser C, Hillegers M, Tiemeier H. Thalamic subregions and obsessive-compulsive symptoms in 2,500 children from the general population. J Am Acad Child Adolesc Psychiatry. 2021;61:321–30.
Jurng J, Park H, Kim T, Park I, Moon S-Y, Lho SK, et al. Smaller volume of posterior thalamic nuclei in patients with obsessive–compulsive disorder. NeuroImage: Clinical. 2021;30:102686.
Iglesias JE, Insausti R, Lerma-Usabiaga G, Bocchetta M, Van Leemput K, Greve DN, et al. A probabilistic atlas of the human thalamic nuclei combining ex vivo MRI and histology. Neuroimage. 2018;183:314–26.
Boedhoe PSW, Schmaal L, Abe Y, Alonso P, Ameis SH, Anticevic A, et al. Cortical abnormalities associated with pediatric and adult obsessive-compulsive disorder: findings from the ENIGMA Obsessive-Compulsive Disorder Working Group. Am J Psychiatry. 2018;175:453–62.
Radua J, Vieta E, Shinohara R, Kochunov P, Quidé Y, Green MJ, et al. Increased power by harmonizing structural MRI site differences with the ComBat batch adjustment method in ENIGMA. Neuroimage. 2020;218:116956.
Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The Yale-Brown obsessive compulsive scale: I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46:1006–11.
Scahill L, Riddle MA, McSwiggin-Hardin M, Ort SI, King RA, Goodman WK, et al. Children’s Yale-Brown obsessive compulsive scale: reliability and validity. J Am Acad Child Adolesc Psychiatry. 1997;36:844–52.
Pergola G, Selvaggi P, Trizio S, Bertolino A, Blasi G. The role of the thalamus in schizophrenia from a neuroimaging perspective. Neurosci Biobehav Rev. 2015;54:57–75.
Anna S, Huang PD, Baxter P, Rogers PD, Julia M, Sheffield PD, et al. Thalamic nuclei volumes in psychotic disorders and in youths with psychosis spectrum symptoms. Am J Psychiatry. 2020;177:1159–67.
Mancini V, Zöller D, Schneider M, Schaer M, Eliez S. Abnormal development and dysconnectivity of distinct thalamic nuclei in patients with 22q11.2 deletion syndrome experiencing auditory hallucinations. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5:875–90.
van den Heuvel OA, Remijnse PL, Mataix-Cols D, Vrenken H, Groenewegen HJ, Uylings HB, et al. The major symptom dimensions of obsessive-compulsive disorder are mediated by partially distinct neural systems. Brain. 2009;132:853–68.
Tau GZ, Peterson BS. Normal development of brain circuits. Neuropsychopharmacology. 2010;35:147–68.
Ziegler G, Hauser TU, Moutoussis M, Bullmore ET, Goodyer IM, Fonagy P, et al. Compulsivity and impulsivity traits linked to attenuated developmental frontostriatal myelination trajectories. Nat Neurosci. 2019;22:992–9.
Tutunji R, El Homsi M, Saaybi S, Al Arab N, Tamim H, Makki M, et al. Thalamic volume and dimensions on MRI in the pediatric population: normative values and correlations: a cross sectional study. Eur J Radiol. 2018;109:27–32.
Sussman D, Leung RC, Chakravarty MM, Lerch JP, Taylor MJ. The developing human brain: age-related changes in cortical, subcortical, and cerebellar anatomy. Brain and Behavior. 2016;6:e00457.
Gilbert AR, Moore GJ, Keshavan MS, Paulson LA, Narula V, Mac Master FP, et al. Decrease in thalamic volumes of pediatric patients with obsessive-compulsive disorder who are taking paroxetine. Arch Gen Psychiatry. 2000;57:449–56.
Mataix-Cols D, Boman M, Monzani B, Rück C, Serlachius E, Långström N, et al. Population-based, multigenerational family clustering study of obsessive-compulsive disorder. JAMA Psychiatry. 2013;70:709–17.
Brander G, Rydell M, Kuja-Halkola R, Fernandez de la Cruz L, Lichtenstein P, Serlachius E, et al. Association of perinatal risk factors with obsessive-compulsive disorder: a population-based birth cohort, sibling control study. JAMA Psychiatry. 2016;73:1135–44.
Van Der Werf YD, Tisserand DJ, Visser PJ, Hofman PAM, Vuurman E, Uylings HBM, et al. Thalamic volume predicts performance on tests of cognitive speed and decreases in healthy aging: a magnetic resonance imaging-based volumetric analysis. Cognitive Brain Res. 2001;11:377–85.
de Jong LW, van der Hiele K, Veer IM, Houwing JJ, Westendorp RGJ, Bollen E, et al. Strongly reduced volumes of putamen and thalamus in Alzheimer’s disease: an MRI study. Brain. 2008;131:3277–85.
Halliday GM. Thalamic changes in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15(Suppl 3):S152–5.
Brennan BP, Rauch SL, Jensen JE, Pope HG Jr. A critical review of magnetic resonance spectroscopy studies of obsessive-compulsive disorder. Biol Psychiatry. 2013;73:24–31.
Bhattacharyya S, Khanna S, Chakrabarty K, Mahadevan A, Christopher R, Shankar SK. Anti-brain autoantibodies and altered excitatory neurotransmitters in obsessive-compulsive disorder. Neuropsychopharmacology. 2009;34:2489–96.
Plitman E, Patel R, Chung JK, Pipitone J, Chavez S, Reyes-Madrigal F, et al. Glutamatergic metabolites, volume and cortical thickness in antipsychotic-naive patients with first-episode psychosis: implications for excitotoxicity. Neuropsychopharmacology. 2016;41:2606–13.
Sairanen M, Lucas G, Ernfors P, Castrén M, Castrén E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci. 2005;25:1089–94.
Post A, Crochemore C, Uhr M, Holsboer F, Behl C. 451. Differential effects of antidepressants on the viability of clonal hippocampal cells. Biol Psychiatry. 2000;47(8, Supplement 1):S138.
Bartholomä P, Erlandsson N, Kaufmann K, Rössler OG, Baumann B, Wirth T, et al. Neuronal cell death induced by antidepressants: lack of correlation with Egr-1, NF-κB and extracellular signal-regulated protein kinase activation. Biochem Pharmacol. 2002;63:1507–16.
Levkovitz Y, Gil-Ad I, Zeldich E, Dayag M, Weizman A. Differential induction of apoptosis by antidepressants in glioma and neuroblastoma cell lines. J Mol Neurosci. 2005;27:29–42.
Schaefer A, Burmann I, Regenthal R, Arélin K, Barth C, Pampel A, et al. Serotonergic modulation of intrinsic functional connectivity. Curr Biol. 2014;24:2314–8.
Acknowledgements
The ENIGMA Obsessive-Compulsive Disorder Working Group gratefully acknowledges support from the NIH BD2K award U54 EB020403 (PI: P.M. Thompson) and Amsterdam Neuroscience (CIA-2019-03-A to O.A. van den Heuvel, Amsterdam Neuroscience Alliance Project to G.A. van Wingen). Supported by the Japan Society for the Promotion of Science (JSPS; KAKENHI Grants No. 18K15523 to Y. Abe, No. 19K03308 to Y. Hirano and A. Nakagawa, No. 16K04342 to A. Nakagawa); National Institute of Mental Health (NIMH; Grant no. 5R01MH116038 to A. Anticevic, No. R01MH085900 to J.D. Feusner, No. K23 MH115206 to P. Gruner, No. R01MH104648 and No. R21MH101441 to R. Marsh, No. R01MH085900 and No. R01MH081864 to J. O’Neill, No. 1R01MH081864 to J. Piacentini, No. K24 MH121571 to C. Pittenger, No. R01 MH074934, No. R21/R33MH107589 and No. R01MH111794 to E.R. Stern, No. R01 MH074934 to D. Tolin); the Alberta Innovates Translational Health Chair in Child and Youth Mental Health and the Ontario Brain Institute funding P.D. Arnold; Swiss National Science Foundation (Grant No. 320030_130237) and the Hartmann Müller Foundation (Grant No. 1460) to S. Brem; Fundação Faculdade de Medicina (Grant No. 86.768 to D.L.C. Costa); the Japan Agency for Medical Research and Development (AMED Brain/MINDS Beyond program Grant No. JP21dm0307002 to Y. Hirano, No. JP21dm0307008 to Y. Sakai); Michael Smith Foundation for Health Research to F. Jaspers-Fayer; the Deutsche Forschungsgemeinschaft (DFG; to D. Rodriguez-Manrique, Grant No. DFG 815/6-1 to N. Kathmann, No. KO 3744/11-1 to K. Koch); The Basic Science Research Program through the National Research Foundation of Korea (NRF) (Grant no. 2019R1A2B5B03100844 to J.S. Kwon); the Marató TV3 Foundation (Grant No. 01/2010 and No. 091710 to L. Lazaro); the National Research Foundation of South Africa to C. Lochner; the Portuguese Foundation for Science and Technology (Fundação para a Ciência e a Tecnologia; FCT; Project No. UIDB/50026/2020 and No. UIDP/50026/2020 to P. Morgado and N. Sousa); the ICVS Scientific Microscopy Platform, member of the national infrastructure PPBI - Portuguese Platform of Bioimaging (Grant No. PPBI-POCI-01-0145-FEDER-022122); the Norte Portugal Regional Operational Programme (NORTE 2020; Grant No. NORTE-01-0145-FEDER-000039 to P. Morgado and N. Sousa), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF); the FLAD Science Award Mental Health 2021 to P. Morgado and N. Sousa; the Government of India grants from the Department of Science and Technology (Grants No. SR/S0/HS/0016/2011 to Y.C. Janardhan Reddy, and DST INSPIRE faculty grant -IFA12-LSBM-26 to J.C. Narayanaswamy) and from the Department of Biotechnology (Grants No. BT/PR13334/Med/30/259/2009 to Y.C. Janardhan Reddy, and No. BT/06/IYBA/2012 to Dr. Janardhanan C. Narayanaswamy, and Accelerator Program for Discovery in Brain Disorders using Stem cells (ADBS), Grant No. BT/PR17316/MED/31/326/2015 to S. Balachander); the Italian Ministry of Health (Grant No. RC19-20-21 to Fabrizio Piras, No. RC18-19-20-21/A to G. Spalletta); the Wellcome Trust-DBT India Alliance (Grant No. 500236/Z/11/Z to G. Venkatasubramanian, Early Career Fellowship Grant No. IA/CPHE/18/1/503956 to. V. Shivakumar); the Carlos III Health Institute (Grant No. PI16/00889 and No. PI19/01171 to C. Soriano-Mas); the Departament de Salut, Generalitat de Catalunya (Grant No. PERIS SLT006/17/249 to C. Soriano-Mas); the Agència de Gestió d’Ajuts Universitaris i de Recerca (Grant No. 2017 SGR 1262 to C. Soriano-Mas); the International OCD Foundation to P.R. Szeszko; the Helse Vest Health Authority (Grant No. 911754 and No. 911880 to A.L. Thorsen); the Netherlands Organisation for Health Research and Development (ZonMw; VIDI Grant No. 91717306 to O.A. van den Heuvel, TOP Grant No. 91211021 to T. White); the South African Medical Research Council (SAMRC) to D.J. Stein; the Dutch Brain Foundation (Nederlandse Hersenstichting; Grant No. HA-2017-00227 to C. Vriend).
Author information
Authors and Affiliations
Consortia
Contributions
Cees J. Weeland: Conceptualization, methodology, formal analysis, resources, data curation, writing—original draft, writing—review and editing, visualization; Selina Kasprzak: Conceptualization, methodology, resources, data curation, writing—original draft, writing—review and editing; Niels T. de Joode, Yoshinari Abe, Pino Alonso, Stephanie H. Ameis, Alan Anticevic, Paul D. Arnold, Srinivas Balachander, Nerisa Banaj, Nuria Bargallo, Marcelo C. Batistuzzo, Francesco Benedetti, Jan C. Beucke, Irene Bollettini, Vilde Brecke, Silvia Brem, Carolina Cappi, Yuqi Cheng, Kang Ik K. Cho, Daniel L.C. Costa, Sara Dallaspezia, Damiaan Denys, Goi Khia Eng, Sónia Ferreira, Jamie D. Feusner, Martine Fontaine, Jean-Paul Fouche, Rachael G. Grazioplene, Patricia Gruner, Mengxin He, Yoshiyuki Hirano, Marcelo Q. Hoexter, Hao Hu, Chaim Huyser, Fern Jaspers-Fayer, Norbert Kathmann, Christian Kaufmann, Minah Kim, Kathrin Koch, Yoo Bin Kwak, Jun Soo Kwon, Luisa Lazaro, Chiang-shan R. Li, Christine Lochner, Rachel Marsh, Ignacio Martínez-Zalacaín, David Mataix-Cols, Jose M. Menchón, Luciano Minnuzi, Pedro Silva Moreira, Pedro Morgado, Akiko Nakagawa, Takashi Nakamae, Janardhanan C. Narayanaswamy, Erika L. Nurmi, Joseph O’Neill, Ana E. Ortiz, Jose C. Pariente, John Piacentini, Maria Picó-Pérez, Federica Piras, Fabrizio Piras, Christopher Pittenger, Y.C. Janardhan Reddy, Daniela Rodriguez-Manrique, Yuki Sakai, Eiji Shimizu, Venkataram Shivakumar, Helen Blair Simpson, Noam Soreni, Carles Soriano-Mas, Nuno Sousa, Gianfranco Spalletta, Emily R. Stern, Michael C. Stevens, S. Evelyn Stewart, Philip R. Szeszko, Jumpei Takahashi, Tais Tanamatis, Anders Lillevik Thorsen, David Tolin, Ysbrand D. van der Werf, Hein van Marle, Guido A. van Wingen, Daniela Vecchio, Venkatasubramanian G., Susanne Walitza, Jicai Wang, Zhen Wang, Anri Watanabe, Lidewij H. Wolters, Xiufeng Xu, Je-Yeon Yun, Qing Zhao and Tonya White: Resources, data curation, writing—review and editing; Paul M. Thompson: Conceptualization, resources, data curation, writing—original draft, writing—review and editing, project administration, funding acquisition; Dan J. Stein: Conceptualization, resources, data curation, writing—original draft, writing—review and editing, project administration; Odile A van den Heuvel: Conceptualization, methodology, resources, data curation, writing—original draft, writing—review and editing, supervision, project administration, funding acquisition; Chris Vriend: Conceptualization, methodology, software, resources, data curation, writing—original draft, writing—review and editing, supervision, project administration, funding acquisition.
Corresponding author
Ethics declarations
Competing interests
Alan Anticevic is a shareholder and member of the technology advisory board for RBNC Therapeutics. Jamie D. Feusner is affiliated with NOCD, LLC; Prof. Mataix-Cols receives personal fees from UpToDate, Inc and Elsevier, both outside the current work; Pedro Morgado has received in the past 3 years grants, CME-related honoraria, or consulting fees from Angelini, AstraZeneca, Bial Foundation, Biogen, DGS-Portugal, FCT, FLAD, Janssen-Cilag, Gulbenkian Foundation, Lundbeck, Springer Healthcare, Tecnimede and 2CA-Braga; Erika L. Nurmi served on the Scientific Advisory Board of Myriad Genetics and Medical Advisory Board of Tourette Association of America and Teva Pharmaceuticals; Christopher Pittenger is a consultant for Biohaven, CH-TAC, Lundbeck (not relevant to this work); in the last 3 years, Dr. Simpson has received research support from Biohaven for a multi-site clinical trial, royalties from Cambridge University Press and UpToDate, Inc, and a stipend from the American Medical Association for serving as Associate Editor of JAMA-Psychiatry; Susanne Walitza received no funds of Industry Outside professional activities and interests are declared under the link of the University of Zurich www.uzh.ch/prof/ssl-dir/interessenbindungen/client/web/. Her work was supported in the last 5 years by the Swiss National Science Foundation (SNF), diverse EU FP7s, Gesundheitsförderung Schweiz, Bfarm Germany, ZInEP, Hartmann Müller Stiftung, Olga Mayenfisch, Gertrud Thalmann, Vontobel, Unicentia, Erika Schwarz Fonds. Paul M. Thompson received partial research support from Biogen, Inc., for research unrelated to this manuscript; Chris Vriend is listed as an inventor on a patent licensed to General Electronic (WO2018115148A1). Odile A. van den Heuvel received a consultation honorarium from Lundbeck. All other authors from the ENIGMA OCD working group have no conflicts of interest related to this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Weeland, C.J., Kasprzak, S., de Joode, N.T. et al. The thalamus and its subnuclei—a gateway to obsessive-compulsive disorder. Transl Psychiatry 12, 70 (2022). https://doi.org/10.1038/s41398-022-01823-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-022-01823-2