Abstract
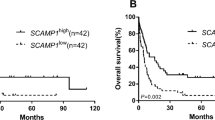
Acute myeloid leukemia (AML) is malignant clonal expansion of myeloid blasts with high heterogeneity and numerous molecular biomarkers have been found to judge the prognosis in some specific classifications of AML. Furthermore, as for patients with cytogenetically normal acute myeloid leukemia (CN-AML), we need to find more new biomarkers to predict the patients’ outcomes. Recently, the expression level of Neuronal Calcium Sensor 1 (NCS1) has been associated with the prognosis of breast cancer and hepatocellular carcinoma, but nothing related has been reported about hematological malignancies. Therefore, we make this study to explore the relationship between the NCS1 expression level and CN-AML. We analyzed the relation between survival and NCS1 RNA expression through 75 CN-AML patients from Cancer Genome Atlas (TCGA) database and 433 CN-AML patients (3 independent datasets) from Gene Expression Omnibus (GEO) database. Additionally, we compared the NCS1 RNA expression between 138 leukemia stem cells positive (LSCs+) samples and 89 leukemia stem cells negative (LSCs−) samples from 78 AML patients from GSE76004 dataset. In our study, CN-AML patients with high expression level of NCS1 have longer EFS or OS. In addition, the NCS1 expression level in leukemia stem cells was low (p = 0.00039). According to these findings, we concluded that the high expression of NCS1 can predict favorable prognosis in CN-AML patients. Furthermore, our work put forward that NCS1 expresses lower in LSCs+, which might be an important mechanism to explain the aggressiveness of AML.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Data openly available in a public repository.
References
O’Donnell MR, Tallman MS, Abboud CN, Altman JK, Appelbaum FR, Arber DA, et al. Acute myeloid leukemia, version 3.2017. J Natl Compr Canc Netw. 2017;15:926–57.
Rollig C, Bornhauser M, Thiede C, Taube F, Kramer M, Mohr B, et al. Long-term prognosis of acute myeloid leukemia according to the new genetic risk classification of the European LeukemiaNet recommendations: evaluation of the proposed reporting system. J Clin Oncol. 2011;29:2758–65.
Arber DA, Orazi A, Hasserjian R. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016;128:462–3.
Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–74.
Milligan DW, Grimwade D, Cullis JO, Bond L, Swirsky D, Craddock C, et al. Guidelines on the management of acute myeloid leukaemia in adults. Br J Haematol. 2006;135:450–74.
Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374:2209–21.
Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. Blood. 1998;92:2322–33.
Santamaria CM, Chillon MC, Garcia-Sanz R, Perez C, Caballero MD, Ramos F, et al. Molecular stratification model for prognosis in cytogenetically normal acute myeloid leukemia. Blood. 2009;114:148–52.
Barragan E, Cervera J, Bolufer P, Ballester S, Martin G, Fernandez P, et al. Prognostic implications of Wilms’ tumor gene (WT1) expression in patients with de novo acute myeloid leukemia. Haematologica. 2004;89:926–33.
Dohner K, Schlenk RF, Habdank M, Scholl C, Rucker FG, Corbacioglu A, et al. Mutant nucleophosmin (NPM1) predicts favorable prognosis in younger adults with acute myeloid leukemia and normal cytogenetics: interaction with other gene mutations. Blood. 2005;106:3740–6.
Heuser M, Beutel G, Krauter J, Dohner K, von Neuhoff N, Schlegelberger B, et al. High meningioma 1 (MN1) expression as a predictor for poor outcome in acute myeloid leukemia with normal cytogenetics. Blood. 2006;108:3898–905.
Marcucci G, Baldus CD, Ruppert AS, Radmacher MD, Mrozek K, Whitman SP, et al. Overexpression of the ETS-related gene, ERG, predicts a worse outcome in acute myeloid leukemia with normal karyotype: a cancer and leukemia group B study. J Clin Oncol. 2005;23:9234–42.
Rockova V, Abbas S, Wouters BJ, Erpelinck CAJ, Beverloo B, Delwel R, et al. Risk stratification of intermediate-risk acute myeloid leukemia: integrative analysis of a multitude of gene mutation and gene expression markers. Blood. 2011;118:1069–76.
Patel JP, Gonen M, Figueroa ME, Fernandez H, Sun ZX, Racevskis J, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366:1079–89.
Lernire S, Jeromin A, Boisselier E. Membrane binding of Neuronal Calcium Sensor-1 (NCS1). Colloid Surf B. 2016;139:138–47.
Bourne Y, Dannenberg J, Pollmann V, Marchot P, Pongs O. Immunocytochemical localization and crystal structure of human frequenin (neuronal calcium sensor 1). J Biol Chem. 2001;276:11949–55.
Moore LM, England A, Ehrlich BE, Rimm DL. Calcium sensor, NCS-1, promotes tumor aggressiveness and predicts patient survival. Mol Cancer Res. 2017;15:942–52.
Zhao XH, Varnai P, Tuymetova G, Balla A, Toth ZE, Oker-Blom C, et al. Interaction of neuronal calcium sensor-1 (NCS-1) with phosphatidylinositol 4-kinase beta stimulates lipid kinase activity and affects membrane trafficking in COS-7 cells. J Biol Chem. 2001;276:40183–9.
Haynes LP, Thomas GMH, Burgoyne RD. Interaction of neuronal calcium sensor-1 and ADP-ribosylation factor 1 allows bidirectional control of phosphatidylinositol 4-kinase beta and trans-Golgi network-plasma membrane traffic. J Biol Chem. 2005;280:6047–54.
Weiss JL, Hui H, Burgoyne RD. Neuronal calcium sensor-1 regulation of calcium channels, secretion, and neuronal outgrowth. Cell Mol Neurobiol. 2010;30:1283–92.
Nakamura TY, Jeromin A, Smith G, Kurushima H, Koga H, Nakabeppu Y, et al. Novel role of neuronal Ca2+ sensor-1 as a survival factor up-regulated in injured neurons. J Cell Biol. 2006;172:1081–91.
Boeckel GR, Ehrlich BE. NCS-1 is a regulator of calcium signaling in health and disease. Biochim Biophys Acta Mol Cell Res. 2018;1865:1660–7.
Dragicevic E, Poetschke C, Duda J, Schlaudraff F, Lammel S, Schiemann J, et al. Cav1.3 channels control D2-autoreceptor responses via NCS-1 in substantia nigra dopamine neurons. Brain. 2014;137:2287–302.
Dahl JP, Jepson C, Levenson R, Wileyto EP, Patterson F, Berrettini WH, et al. Interaction between variation in the D2 dopamine receptor (DRD2) and the neuronal calcium sensor-1 (FREQ) genes in predicting response to nicotine replacement therapy for tobacco dependence. Pharmacogenomics J. 2006;6:194–9.
Handley MTW, Lian LY, Haynes LP, Burgoyne RD. Structural and functional deficits in a neuronal calcium sensor-1 mutant identified in a case of autistic spectrum disorder. PLoS ONE. 2010;5:e10534.
Koh PO, Undie AS, Kabbani N, Levenson R, Goldman-Rakic PS, Lidow MS. Up-regulation of neuronal calcium sensor-1 (NCS-1) in the prefrontal cortex of schizophrenic and bipolar patients. Proc Natl Acad Sci USA. 2003;100:313–7.
Schuette D, Moore LM, Robert ME, Taddei TH, Ehrlich BE. Hepatocellular carcinoma outcome is predicted by expression of neuronal calcium sensor 1. Cancer Epidemiol Biomarkers Prev. 2018;27:1091–1100.
Metzeler KH, Hummel M, Bloomfield CD, Spiekermann K, Braess J, Sauerland MC, et al. An 86-probe-set gene-expression signature predicts survival in cytogenetically normal acute myeloid leukemia. Blood. 2008;112:4193–201.
Kharas MG, Lengner CJ, Al-Shahrour F, Bullinger L, Ball B, Zaidi S, et al. Musashi-2 regulates normal hematopoiesis and promotes aggressive myeloid leukemia. Nat Med. 2010;16:903–8.
Chuang MK, Chiu YC, Chou WC, Hou HA, Tseng MH, Kuo YY, et al. An mRNA expression signature for prognostication in de novo acute myeloid leukemia patients with normal karyotype. Oncotarget. 2015;6:39098–110.
Ng SW, Mitchell A, Kennedy JA, Chen WC, McLeod J, Ibrahimova N, et al. A 17-gene stemness score for rapid determination of risk in acute leukaemia. Nature. 2016;540:433–7.
Darwish NHE, Sudha T, Godugu K, Elbaz O, Abdelghaffar HA, Hassan EEA, et al. Acute myeloid leukemia stem cell markers in prognosis and targeted therapy: potential impact of BMI-1, TIM-3 and CLL-1. Oncotarget. 2016;7:57811–20.
Hope KJ, Jin LQ, Dick JE. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nat Immunol. 2004;5:738–43.
Wang JCY, Dick JE. Cancer stem cells: lessons from leukemia. Trends Cell Biol. 2005;15:494–501.
Yabushita T, Satake H, Maruoka H, Morita M, Katoh D, Shimomura Y, et al. Expression of multiple leukemic stem cell markers is associated with poor prognosis in de novo acute myeloid leukemia. Leuk Lymphoma. 2018;59:2144–51.
Acknowledgements
The authors thank datasets GSE12417, GSE22778, GSE71014, GSE76004 from GEO database and dataset from TCGA database.
Funding
This work was funded by National Natural Science Foundation of China (81800195), Key Clinical Projects of Peking University Third Hospital (BYSYZD2019026, BYSYDL2021006), interdisciplinary medicine Seed Fund of Peking University (BMU2018MB004), Natural Science Foundation of Beijing Municipality (7182178 and 7132183), China Health Promotion Foundation (CHPF-zlkysx-001) and Wu Jieping Medical Foundation (320.6750).
Author information
Authors and Affiliations
Contributions
HJ, XZ, XH and CY conceived the project. WZ, JW and WL analyzed the data. WZ, JW, WL, XL, YZ, PY, MZ, KH, SL, GD, CY, XH, XZ and HJ contributed toward the interpretation of the data. All authors wrote and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, W., Wang, J., Li, W. et al. The expression level of Neuronal Calcium Sensor 1 can predict the prognosis of cytogenetically normal AML. Pharmacogenomics J 23, 89–94 (2023). https://doi.org/10.1038/s41397-023-00301-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41397-023-00301-2