Abstract
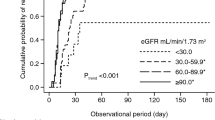
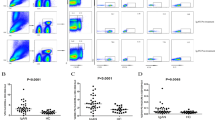
Steroid remains the keystone therapy for Idiopathic Nephrotic Syndrome (NS). Besides genetic factors and histological changes, pharmacogenomic factors also affect the steroid response. The upregulation of P-glycoprotein (P-gp) and Multidrug resistance-associated protein 1 (MRP-1) modulate the pharmacokinetics of steroids and may contribute to steroid resistance. Flow-cytometric analysis of P-gp, MRP-1 expression and functional activity on peripheral blood mononuclear cells (PBMCs) was carried out in steroid-sensitive nephrotic syndrome (SSNS) (n = 171, male 103, mean age = 8.54 ± 4.3); and steroid-resistant nephrotic syndrome (SRNS) (n = 83, male 43, mean age = 7.43 ± 4.6) patients. The genotypings of MDR-1 gene were carried out using PCR-RFLP. We observed that the percentage expression of P-gp (10.01 ± 2.09 and 3.79 ± 1.13, p < 0.001); and MRP-1 (15.91 ± 3.99 and 7.40 ± 2.33, p < 0.001) on lymphocyte gated population were significantly higher in SRNS than that of SSNS. The functional activity of P-gp and MRP-1 was also significantly escalated in SRNS as compared to SSNS (68.10 ± 13.35 and 28.93 ± 7.57, p < 0.001); (72.13 ± 8.34 and 31.56 ± 8.65, p < 0.001) respectively. AUC-ROC curve analysis revealed that P-gp and MRP-1 expression with a cut-off value of 7.13% and 9.62% predicted SRNS with the sensitivity of 90% and 80.7%; and specificity 90% and 80%, respectively. Moreover, MDR-1 homozygous mutant TT+AA for G2677T/A (rs2032582) was significantly associated with SRNS (p = 0.025, OR = 2.86 CI = 1.14–7.14). The expression of P-gp (9.68 ± 4.99 v/s 5.88 ± 3.38, p = 0.002) was significantly higher in the patients of homozygous mutant alleles compared to wildtype GG. The increased expression and functionality of P-gp and MRP-1 contribute to steroid resistance, and MDR-1 homozygous mutant G2677T/A promotes steroid resistance by inducing P-gp expression in NS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
McBryde KD, Kershaw DB, Smoyer WE. Pediatric steroid-resistant nephrotic syndrome. Curr Probl Pediatr Adolesc Health Care. 2001;31:280–307.
Frank C, Herrmann M, Fernandez S, Dirnecker D, Boswald M, Kolowos W, et al. Dominant T cells in idiopathic nephrotic syndrome of childhood. Kidney Int. 2000;57:510–7.
Colucci M, Corpetti G, Emma F, Vivarelli M. Immunology of idiopathic nephrotic syndrome. Pediatr Nephrol. 2018;33:573–84.
Prasad N, Jaiswal AK, Agarwal V, Yadav B, Sharma RK, Rai M, et al. Differential alteration in peripheral T-regulatory and T-effector cells with change in P-glycoprotein expression in Childhood Nephrotic Syndrome: a longitudinal study. Cytokine. 2015;72:190–6.
Liu LL, Qin Y, Cai JF, Wang HY, Tao JL, Li H, et al. Th17/Treg imbalance in adult patients with minimal change nephrotic syndrome. Clin Immunol. 2011;139:314–20.
Singh VK, Mehrotra S, Agarwal SS. The paradigm of Th1 and Th2 cytokines: its relevance to autoimmunity and allergy. Immunol Res. 1999;20:147–61.
Primary nephrotic syndrome in children: clinical significance of histopathologic variants of minimal change and of diffuse mesangial hypercellularity. A Report of the International Study of Kidney Disease in Children. Kidney Int. 1981;20:765–71.
Goodwin JE. Role of the glucocorticoid receptor in glomerular disease. Am J Physiol Ren Physiol. 2019;317:F133–F6.
Hebbar PB, Archer TK. Chromatin remodeling by nuclear receptors. Chromosoma. 2003;111:495–504.
McKay LI, Cidlowski JA. Molecular control of immune/inflammatory responses: interactions between nuclear factor-kappa B and steroid receptor-signaling pathways. Endocr Rev. 1999;20:435–59.
Tullus K, Webb H, Bagga A. Management of steroid-resistant nephrotic syndrome in children and adolescents. Lancet Child Adolesc Health. 2018;2:880–90.
Kansal A, Tripathi D, Rai MK, Agarwal V. Persistent expression and function of P-glycoprotein on peripheral blood lymphocytes identifies corticosteroid resistance in patients with systemic lupus erythematosus. Clin Rheumatol. 2016;35:341–9.
Badr HS, El-Hawy MA, Helwa MA. P-Glycoprotein Activity in Steroid-Responsive vs. Steroid-Resistant Nephrotic Syndrome. Indian J Pediatr. 2016;83:1222–6.
Wasilewska AM, Zoch-Zwierz WM, Pietruczuk M. Expression of P-glycoprotein in lymphocytes of children with nephrotic syndrome treated with glucocorticoids. Eur J Pediatr. 2006;165:839–44.
Walsh N, Larkin A, Kennedy S, Connolly L, Ballot J, Ooi W, et al. Expression of multidrug resistance markers ABCB1 (MDR-1/P-gp) and ABCC1 (MRP-1) in renal cell carcinoma. BMC Urol. 2009;9:6.
Muller-Deile J, Schiffer M. Podocyte directed therapy of nephrotic syndrome-can we bring the inside out? Pediatr Nephrol. 2016;31:393–405.
Parasrampuria DA, Lantz MV, Birnbaum JL, Vincenti FG, Benet LZ. Effect of calcineurin inhibitor therapy on P-gp expression and function in lymphocytes of renal transplant patients: a preliminary evaluation. J Clin Pharm. 2002;42:304–11.
Kara A, Gurgoze MK, Kara M, Aydin M. Evaluation of Genetic Polymorphisms for Determining Steroid Response in Nephrotic Children. Ann Clin Lab Sci. 2018;48:478–83.
Jafar T, Prasad N, Agarwal V, Mahdi A, Gupta A, Sharma RK, et al. MDR-1 gene polymorphisms in steroid-responsive versus steroid-resistant nephrotic syndrome in children. Nephrol Dial Transpl. 2011;26:3968–74.
Prasad S, Tripathi D, Rai MK, Aggarwal S, Mittal B, Agarwal V. Multidrug resistance protein-1 expression, function and polymorphisms in patients with rheumatoid arthritis not responding to methotrexate. Int J Rheum Dis. 2014;17:878–86.
Youssef DM, Elbehidy RM, Abdelhalim HS, Amr GE. Soluble interleukine-2 receptor and MDR1 gene expression levels as inflammatory biomarkers for prediction of steroid response in children with nephrotic syndrome. Iran J Kidney Dis. 2011;5:154–61.
Funaki S, Takahashi S, Wada N, Murakami H, Harada K. Multiple drug-resistant gene 1 in children with steroid-sensitive nephrotic syndrome. Pediatr Int. 2008;50:159–61.
Cole SP, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, et al. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992;258:1650–4.
Flens MJ, Zaman GJ, van der Valk P, Izquierdo MA, Schroeijers AB, Scheffer GL, et al. Tissue distribution of the multidrug resistance protein. Am J Pathol. 1996;148:1237–47.
Stride BD, Valdimarsson G, Gerlach JH, Wilson GM, Cole SP, Deeley RG. Structure and expression of the messenger RNA encoding the murine multidrug resistance protein, an ATP-binding cassette transporter. Mol Pharm. 1996;49:962–71.
Peng KC, Cluzeaud F, Bens M, Duong Van Huyen JP, Wioland MA, Lacave R, et al. Tissue and cell distribution of the multidrug resistance-associated protein (MRP) in mouse intestine and kidney. J Histochem Cytochem. 1999;47:757–68.
Valera ET, Scrideli CA, Queiroz RG, Mori BM, Tone LG. Multiple drug resistance protein (MDR-1), multidrug resistance-related protein (MRP) and lung resistance protein (LRP) gene expression in childhood acute lymphoblastic leukemia. Sao Paulo Med J. 2004;122:166–71.
den Boer ML, Pieters R, Kazemier KM, Janka-Schaub GE, Henze G, Veerman AJ. The modulating effect of PSC 833, cyclosporin A, verapamil and genistein on in vitro cytotoxicity and intracellular content of daunorubicin in childhood acute lymphoblastic leukemia. Leukemia. 1998;12:912–20.
Singh H, Prasad N, Misra DP, Jaiswal AK, Agarwal V. P-glycoprotein and/or Histone Deacetylase 2 Regulates Steroid Responsiveness in Childhood Nephrotic Syndrome. Indian. J Rheumatol. 2020;15:5–10.
Chowdhary VR. When doing the right thing is wrong–drug efflux pumps in steroid-resistant nephrotic syndrome. Indian J Rheumatol. 2020;15:1.
Cizmarikova M, Podracka L, Klimcakova L, Habalova V, Boor A, Mojzis J, et al. MDR1 polymorphisms and idiopathic nephrotic syndrome in Slovak children: preliminary results. Med Sci Monit. 2015;21:59–68.
Belliard AM, Tardivel S, Farinotti R, Lacour B, Leroy C. Effect of hr-IL2 treatment on intestinal P-glycoprotein expression and activity in Caco-2 cells. J Pharm Pharm. 2002;54:1103–9.
Gulati S, Prasad N, Sharma RK, Kumar A, Gupta A, Baburaj VP. Tacrolimus: a new therapy for steroid-resistant nephrotic syndrome in children. Nephrol Dial Transpl. 2008;23:910–3.
Kim HG, Hien TT, Han EH, Hwang YP, Choi JH, Kang KW, et al. Metformin inhibits P-glycoprotein expression via the NF-kappaB pathway and CRE transcriptional activity through AMPK activation. Br J Pharm. 2011;162:1096–108.
Acknowledgements
We acknowledge the research grant of Indian Council of Medical Research for the completion of the project GIA/40/2014-DHR.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Prasad, N., Singh, H., Jaiswal, A. et al. Overexpression of P-glycoprotein and MRP-1 are pharmacogenomic biomarkers to determine steroid resistant phenotype in childhood idiopathic nephrotic syndrome. Pharmacogenomics J 21, 566–573 (2021). https://doi.org/10.1038/s41397-021-00233-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41397-021-00233-9