Abstract
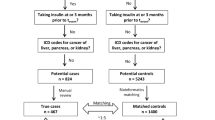
The demonstration of the link between certain genetic variations and drug response has allowed the emergence of pharmacogenetics, which offers many opportunities to improve patient care. Type-2 diabetes mellitus is a disease for which several gene polymorphisms have been reported to be associated with drug response. Sulfonylureas are commonly used for the management of this disease. Genetic polymorphisms of CYP2C9, the main enzyme involved in the metabolism of sulfonylureas, have been associated with the risk of severe hypoglycaemia, particularly in poor metabolizers carrying CYP2C9 *3/*3 genotype, and especially in the case of patients treated with glimepiride. The objectives of the present study were to evaluate the potential clinical and economic outcomes of using CYP2C9 genotype data to guide the management of SU regimen in patients initiating glimepiride therapy, and to identify factors affecting the cost-effectiveness of this treatment scheme. The analysis was conducted using a decision tree, considering a 1-year time horizon, and taking as perspective that of the French national health insurance system. With pharmacogenetic-guided therapy, the cost to avoid an episode of severe hypoglycaemia event per 100 000 patients treated was €421 834. Genotyping cost was the most influential factor on the incremental cost-effectiveness ratio. In conclusion, the potential cost of CYP2C9 genotype-guided dosing for glimepiride therapy is relatively high, and associated with modest improvements with respect to the number of hypoglycaemia avoided, as compared with standard dosing. Additional economic studies are required to better specify the usefulness of CYP2C9 genotyping prior to glimepiride regimen initiation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Allorge D, Loriot M-A. La pharmacogénétique ou la promesse d’une médecine personnalisée: variations du métabolisme et du transport des médicaments. Ann Biol Clin (Paris). 2004;62:499–511.
Charbonnel B, Simon D, Dallongeville J, Bureau I, Gourmelen J, Detournay B. Coût du diabète de type 2 en France: une analyse des données de l’EGB. Médecine Mal Métaboliques. 2017;11:IIS24–7. https://doi.org/10.1016/S1957-2557(18)30027-0
Inserm. Diabète de type 2. 2014. https://www.inserm.fr/information-en-sante/dossiers-information/diabete-type-2. Accessed April 11, 2018.
Piffaretti C, Fagot-Campagna A, Rey G, Antero-Jacquemin J, Latouche A, Mandereau-Bruno L, et al. Déterminants de la mortalité des personnes diabétiques de type 2. Cohortes Entred, France, 2002–2013;2016.
Halimi S. Hypoglycémie 2002. http://www-sante.ujf-grenoble.fr/SANTE/corpus/disciplines/endoc/nutrition/206/lecon206.htm Accessed April 11, 2018.
Cariou B, Fontaine P, Eschwege E, Lièvre M, Gouet D, Huet D, et al. Frequency and predictors of confirmed hypoglycaemia in type 1 and insulin-treated type 2 diabetes mellitus patients in a real-life setting: results from the DIALOG study. Diabetes Metab. 2015;41:116–25. https://doi.org/10.1016/j.diabet.2014.10.007
Sultan A. Hypoglycémie et risque cardiovasculaire dans le diabète de type 1 et de type 2. Médecine Mal Métaboliques. 2016;10:13–4.
Grimaldi A, Slama G, Tubiana-Rufi N, Heurtier A, Selam JL, Scheen A, et al. L’hypoglycémie du patient diabétique. Diabetes Metab. 1997;23:100–8.
Harris S, Mamdani M, Galbo-Jørgensen CB, Bøgelund M, Gundgaard J, Groleau D. The effect of hypoglycemia on health-related quality of life: Canadian results from a multinational time trade-off survey. Can J Diabetes. 2014;38:45–52. https://doi.org/10.1016/j.jcjd.2013.09.001
Detournay B, Halimi S, Torreton E, Levy P. Coût des hospitalisations pour hypoglycémie en France chez les patients diabétiques de type 2: étude fondée sur le PMSI et la base EGB de la CNAMTS. Médecine Mal Métaboliques. 2017;11:IIS16–9. https://doi.org/10.1016/S1957-2557(18)30025-7
Aquilante CL. Sulfonylurea pharmacogenomics in Type 2 diabetes: the influence of drug target and diabetes risk polymorphisms. Expert Rev Cardiovasc Ther. 2010;8:359–72. https://doi.org/10.1586/erc.09.154
Glasser L, Alt-Tebacher M, Schlienger J-L. Hypoglycémies iatrogènes: Iatrogenic hypoglycemias. Médecine Mal Métaboliques. 2011;5:377–81. https://doi.org/10.1016/S1957-2557(11)70269-3
van Leeuwen N, Swen JJ, Guchelaar H-J, ’t Hart LM. The role of pharmacogenetics in drug disposition and response of oral glucose-lowering drugs. Clin Pharmacokinet. 2013;52:833–54. https://doi.org/10.1007/s40262-013-0076-3
Leonard CE, Han X, Brensinger CM, Bilker WB, Cardillo S, Flory JH, et al. Comparative risk of serious hypoglycemia with oral antidiabetic monotherapy: A retrospective cohort study. Pharmacoepidemiol Drug Saf. 2018;27:9–18. https://doi.org/10.1002/pds.4337
Pink J, Pirmohamed M, Lane S, Hughes DA. Cost-effectiveness of pharmacogenetics-guided warfarin therapy vs. alternative anticoagulation in atrial fibrillation. Clin Pharm Ther. 2014;95:199–207. https://doi.org/10.1038/clpt.2013.190
You JHS, Chan FWH, Wong RSM, Cheng G. The potential clinical and economic outcomes of pharmacogenetics-oriented management of warfarin therapy - a decision analysis. Thromb Haemost. 2004;92:590–7. https://doi.org/10.1160/TH04-03-0161
Winter J, Walker A, Shapiro D, Gaffney D, Spooner RJ, Mills PR. Cost-effectiveness of thiopurine methyltransferase genotype screening in patients about to commence azathioprine therapy for treatment of inflammatory bowel disease. Aliment Pharm Ther. 2004;20:593–9. https://doi.org/10.1111/j.1365-2036.2004.02124.x
Thompson AJ, Newman WG, Elliott RA, Roberts SA, Tricker K, Payne K. The cost-effectiveness of a pharmacogenetic test: a trial-based evaluation of TPMT genotyping for azathioprine. Value Health. 2014;17:22–33. https://doi.org/10.1016/j.jval.2013.10.007
Butzke B, Oduncu FS, Severin F, Pfeufer A, Heinemann V, Giessen-Jung C, et al. The cost-effectiveness of UGT1A1 genotyping before colorectal cancer treatment with irinotecan from the perspective of the German statutory health insurance. Acta Oncol. 2016;55:318–28. https://doi.org/10.3109/0284186X.2015.1053983
Pichereau S, Le Louarn A, Lecomte T, Blasco H, Le Guellec C, Bourgoin H. Cost-effectiveness of UGT1A1*28 genotyping in preventing severe neutropenia following FOLFIRI therapy in colorectal cancer. J Pharm Pharm Sci. 2010;13:615–25.
Roncato R, Cecchin E, Montico M, De Mattia E, Giodini L, Buonadonna A, et al. Cost evaluation of irinotecan-related toxicities associated with the UGT1A1*28 patient genotype. Clin Pharm Ther. 2017;102:123–30. https://doi.org/10.1002/cpt.615
Deiman BALM, Tonino PAL, Kouhestani K, Schrover CEM, Scharnhorst V, Dekker LRC, et al. Reduced number of cardiovascular events and increased cost-effectiveness by genotype-guided antiplatelet therapy in patients undergoing percutaneous coronary interventions in the Netherlands. Neth Heart J. 2016;24:589–99. https://doi.org/10.1007/s12471-016-0873-z
Mitropoulou C, Fragoulakis V, Rakicevic LB, Novkovic MM, Vozikis A, Matic DM, et al. Economic analysis of pharmacogenomic-guided clopidogrel treatment in Serbian patients with myocardial infarction undergoing primary percutaneous coronary intervention. Pharmacogenomics. 2016. https://doi.org/10.2217/pgs-2016-0052
Herbild L, Bech M, Gyrd-Hansen D, Christensen M, Werge T, Nielsen KA. Do guidelines recommending pharmacogenetic testing of psychiatric patients affect treatment costs and the use of healthcare services? Scand J Public Health. 2011;39:147–55. https://doi.org/10.1177/1403494810393300
Herbild L, Andersen SE, Werge T, Rasmussen HB, Jürgens G. Does pharmacogenetic testing for CYP450 2D6 and 2C19 among patients with diagnoses within the schizophrenic spectrum reduce treatment costs? Basic Clin Pharm Toxicol. 2013;113:266–72. https://doi.org/10.1111/bcpt.12093
Martin MA, Kroetz DL. Abacavir pharmacogenetics – from initial reports to standard of care. Pharmacotherapy. 2013;33:765–75. https://doi.org/10.1002/phar.1278
Allorge D, Beaune P-H, Becquemont L, Bessard G, Bezieau S, Boisdron-Celle M, et al. La pharmacogénétique moléculaire hospitalière en France: données actuelles et perspectives. Ann Pharm Fr. 2007;65:371–81. https://doi.org/10.1016/S0003-4509(07)74196-2
Haute Autorité de Santé. Stratégie médicamenteuse du contrôle glycémique du diabète de type 2. 2013.
Faure S. Sulfamides hypoglycémiants. Actual Pharm. 2011;50:53–6. https://doi.org/10.1016/S0515-3700(11)71029-1
Darmon P, Bauduceau B, Bordier L, Bringer J, Chabrier G, Charbonnel B, et al. Prise de position de la Société Francophone du Diabète (SFD) sur la prise en charge médicamenteuse de l’hyperglycémie du patient diabétique de type 2. Médecine Mal Métaboliques. 2017;11:577–93. https://doi.org/10.1016/S1957-2557(17)30139-6
Wiley LK, Vanhouten JP, Samuels DC, Aldrich MC, Roden DM, Peterson JF, et al. Strategies for equitable pharmacogenomic-guided warfarin dosing among European and African American individuals in a clinical population. Pac Symp Biocomput. 2017;22:545–56. https://doi.org/10.1142/9789813207813_0050
Li X, Liu R, Yan H, Tang J, Yin J-Y, Mao X-Y, et al. Effect of CYP2C9-VKORC1 interaction on warfarin stable dosage and its predictive algorithm. J Clin Pharm. 2015;55:251–7. https://doi.org/10.1002/jcph.392
Yasar U, Eliasson E, Dahl ML, Johansson I, Ingelman-Sundberg M, Sjöqvist F. Validation of methods for CYP2C9 genotyping: frequencies of mutant alleles in a Swedish population. Biochem Biophys Res Commun. 1999;254:628–31. https://doi.org/10.1006/bbrc.1998.9992
Holstein A, Hahn M, Patzer O, Seeringer A, Kovacs P, Stingl J. Impact of clinical factors and CYP2C9 variants for the risk of severe sulfonylurea-induced hypoglycemia. Eur J Clin Pharm. 2011;67:471–6. https://doi.org/10.1007/s00228-010-0976-1
Schloot NC, Haupt A, Schütt M, Badenhoop K, Laimer M, Nicolay C, et al. Risk of severe hypoglycemia in sulfonylurea-treated patients from diabetes centers in Germany/Austria: how big is the problem? Which patients are at risk? Diabetes Metab Res Rev. 2016;32:316–24. https://doi.org/10.1002/dmrr.2722
Detournay B, Halimi S, Robert J, Deschaseaux C, Dejager S. Hypoglycemia hospitalization frequency in patients with type 2 diabetes mellitus: a comparison of dipeptidyl peptidase 4 inhibitors and insulin secretagogues using the French health insurance database. Vasc Health Risk Manag. 2015;11:417–25. https://doi.org/10.2147/VHRM.S84507
Acknowledgements
The authors thank Dr. Myriam Moret and Dr. Michel Bensimon for help in the obtention of the clinical parameters of the study. The authors also thank Philip Robinson and Verena Landel (DRCI, Hospices Civils de Lyon) for help in manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fokoun, C., Serrier, H., Rabier, H. et al. Pharmacogenetic-guided glimepiride therapy in type-2 diabetes mellitus: a cost-effectiveness study. Pharmacogenomics J 21, 559–565 (2021). https://doi.org/10.1038/s41397-021-00232-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41397-021-00232-w
This article is cited by
-
Therapeutic potential of pyrrole and pyrrolidine analogs: an update
Molecular Diversity (2022)