Abstract
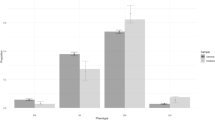
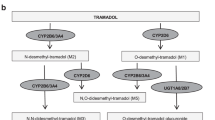
Cytochrome P450 2D6 (CYP2D6) O-demethylates codeine to the active drug, morphine. However, the utility of testing for CYP2D6 metabolizer status in patients receiving codeine in real-world clinical practice is poorly defined. Using data from a DNA bank linked to de-identified electronic health records, we studied 157 patients with a baseline pain score higher than 4 (0–10 scale) who received codeine. Based on CYP2D6 genotyping, 69 were classified as poor/intermediate and 88 as normal/ultrarapid CYP2D6 metabolizers. Pain response was defined as a score of 4 or lower while receiving codeine. In a propensity-score adjusted model, poor/intermediate metabolizers had lower odds (OR = 0.35, p = 0.02) of achieving a pain response than normal/ultrarapid metabolizers. To discriminate between codeine responders and nonresponders, a score including CYP2D6 phenotype and clinical variables was built. The response rate was 38.5% among patients in the high, 17.3% in the intermediate, and 9.4% in the low-score groups, respectively (p = 0.001).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Forbes K. Pain in patients with cancer: the World Health Organization analgesic ladder and beyond. Clin Oncol. 2011;23:379–80.
Crews KR, Gaedigk A, Dunnenberger HM, Klein TE, Shen DD, Callaghan JT, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for codeine therapy in the context of cytochrome P450 2D6 (CYP2D6) genotype. Clin Pharm Ther. 2012;91:321–6.
Kirchheiner J, Schmidt H, Tzvetkov M, Keulen JT, Lotsch J, Roots I, et al. Pharmacokinetics of codeine and its metabolite morphine in ultra-rapid metabolizers due to CYP2D6 duplication. Pharmacogenomics J. 2007;7:257–65.
Nofziger C, Turner AJ, Sangkuhl K, Whirl-Carrillo M, Agundez JAG, Black JL, et al. PharmVar GeneFocus: CYP2D6. Clin Pharm Ther. 2020;107:154–70.
Poulsen L, Brosen K, Arendt-Nielsen L, Gram LF, Elbaek K, Sindrup SH. Codeine and morphine in extensive and poor metabolizers of sparteine: pharmacokinetics, analgesic effect and side effects. Eur J Clin Pharm. 1996;51:289–95.
Consortium CPI. CPIC guideline for codeine and CYP2D6. 2020. Available from: https://cpicpgx.org/guidelines/guideline-for-codeine-and-cyp2d6/.
Caudle KE, Sangkuhl K, Whirl-Carrillo M, Swen JJ, Haidar CE, Klein TE, et al. Standardizing CYP2D6 genotype to phenotype translation: Consensus Recommendations from the Clinical Pharmacogenetics Implementation Consortium and Dutch Pharmacogenetics Working Group. Clin Transl Sci.2020;13:116–24.
Desmeules J, Gascon MP, Dayer P, Magistris M. Impact of environmental and genetic factors on codeine analgesia. Eur J Clin Pharm. 1991;41:23–6.
Pulley J, Clayton E, Bernard GR, Roden DM, Masys DR. Principles of human subjects protections applied in an opt-out, de-identified biobank. Clin Transl Sci. 2010;3:42–8.
Roden DM, Pulley JM, Basford MA, Bernard GR, Clayton EW, Balser JR, et al. Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clin Pharm Ther. 2008;84:362–9.
Wei WQ, Denny JC. Extracting research-quality phenotypes from electronic health records to support precision medicine. Genome Med. 2015;7:41.
Kannampallil T, Galanter WL, Falck S, Gaunt MJ, Gibbons RD, McNutt R, et al. Characterizing the pain score trajectories of hospitalized adult medical and surgical patients: a retrospective cohort study. Pain. 2016;157:2739–46.
Woo A, Lechner B, Fu T, Wong CS, Chiu N, Lam H, et al. Cut points for mild, moderate, and severe pain among cancer and non-cancer patients: a literature review. Ann Palliat Med. 2015;4:176–83.
FDA. Drug development and drug interactions: table of substrates, inhibitors and inducers. 2020. Available from: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/DrugInteractionsLabeling/ucm093664.htm#classInhibit.
PharmGKB. Gene-specific information tables for CYP2D6. 2020.Available from: https://www.pharmgkb.org/page/cyp2d6RefMaterials.
Dupont WD, Plummer WD Jr. PS power and sample size program available for free on the internet. Control Clin Trials. 1997;18:274.
Smith DM, Weitzel KW, Elsey AR, Langaee T, Gong Y, Wake DT, et al. CYP2D6-guided opioid therapy improves pain control in CYP2D6 intermediate and poor metabolizers: a pragmatic clinical trial. Genet Med. 2019;21:1842–50.
Saravanakumar A, Sadighi A, Ryu R, Akhlaghi F. Physicochemical properties, biotransformation, and transport pathways of established and newly approved medications: a systematic review of the top 200 most prescribed drugs vs. the FDA-approved drugs between 2005 and 2016. Clin Pharmacokinet. 2019;58:1281–94.
Wang B, Yang LP, Zhang XZ, Huang SQ, Bartlam M, Zhou SF. New insights into the structural characteristics and functional relevance of the human cytochrome P450 2D6 enzyme. Drug Metab Rev. 2009;41:573–643.
Roden DM, Van Driest SL, Mosley JD, Wells QS, Robinson JR, Denny JC, et al. Benefit of preemptive pharmacogenetic information on clinical outcome. Clin Pharmacol Ther. 2018;103:787–94.
Olsen MF, Bjerre E, Hansen MD, Hilden J, Landler NE, Tendal B, et al. Pain relief that matters to patients: systematic review of empirical studies assessing the minimum clinically important difference in acute pain. BMC Med. 2017;15:35.
Kelly AM. The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emerg Med J. 2001;18:205–7.
Acknowledgements
This study was supported by grants R01GM109145 and R35GM131770. CPC and ALD were also supported by grant R01AR073764 and the Veterans Health Administration Merit Award 1I01CX001741. The data set(s) used for the analyses described were obtained from Vanderbilt University Medical Center’s BioVU, which is supported by numerous sources: institutional funding, private agencies, and federal grants. These include the NIH funded Shared Instrumentation grant S10RR025141; and CTSA grants UL1TR002243, UL1TR000445, and UL1RR024975. Genomic data are also supported by investigator-led projects that include U01HG004798, R01NS032830, RC2GM092618, P50GM115305, U01HG006378, U19HL065962, R01HD074711; and additional funding sources are listed at https://victr.vumc.org/biovu/index.html?sid=229. The funding sources had no role in the collection, analysis, or interpretation of data, writing of the paper, or decision to submit for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Carranza-Leon, D., Dickson, A.L., Gaedigk, A. et al. CYP2D6 genotype and reduced codeine analgesic effect in real-world clinical practice. Pharmacogenomics J 21, 484–490 (2021). https://doi.org/10.1038/s41397-021-00226-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41397-021-00226-8
This article is cited by
-
Pharmacogenomics: current status and future perspectives
Nature Reviews Genetics (2023)
-
Cytochrome P450-2D6 activity in people with codeine use disorder
The Pharmacogenomics Journal (2023)