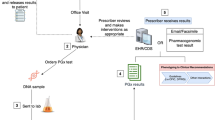
Abstract
Effective doctor–patient communication is critical for disease management, especially when considering genetic information. We studied patient-provider communications after implementing a point-of-care pharmacogenomic results delivery system to understand whether pharmacogenomic results are discussed and whether medication recall is impacted. Outpatients undergoing preemptive pharmacogenomic testing (cases), non-genotyped controls, and study providers were surveyed from October 2012–May 2017. Patient responses were compared between visits where pharmacogenomic results guided prescribing versus visits where pharmacogenomics did not guide prescribing. Provider knowledge of pharmacogenomics, before and during study participation, was also analyzed. Both providers and case patients frequently reported discussions of genetic results after visits where pharmacogenomic information guided prescribing. Importantly, medication changes from visits where pharmacogenomics influenced prescribing were more often recalled than non-pharmacogenomic guided medication changes (OR = 3.3 [1.6–6.7], p = 0.001). Case patients who had separate visits where pharmacogenomics did and did not, respectively, influence prescribing more often remembered medication changes from visits where genomic-based guidance was used (OR = 3.4 [1.2–9.3], p = 0.02). Providers also displayed dramatic increases in personal genomic understanding through program participation (94% felt at least somewhat informed about pharmacogenomics post-participation, compared to 61% at baseline, p = 0.04). Using genomic information during prescribing increases patient-provider communications, patient medication recall, and provider understanding of genomics, important ancillary benefits to clinical use of pharmacogenomics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ha JF, Longnecker N. Doctor–patient communication: a review. Ochsner J. 2010;10:38–43.
Bredart A, Bouleuc C, Dolbeault S. Doctor–patient communication and satisfaction with care in oncology. Curr Opin Oncol. 2005;17:351–4.
Herndon JH, Pollick KJ. Continuing concerns, new challenges, and next steps in physician–patient communication. J Bone Jt Surg Am. 2002;84-A:309–15.
Zolnierek KB, Dimatteo MR. Physician communication and patient adherence to treatment: a meta-analysis. Med Care. 2009;47:826–34.
Stewart MA. Effective physician–patient communication and health outcomes: a review. CMAJ. 1995;152:1423–33.
Duffy FD, Gordon GH, Whelan G, Cole-Kelly K, Frankel R, Buffone N, et al. Assessing competence in communication and interpersonal skills: the Kalamazoo II report. Acad Med. 2004;79:495–507.
Tongue JR, Epps HR, Forese LL. Communication skills. Instr Course Lect. 2005;54:3–9.
Taran S. An examination of the factors contributing to poor communication outside the physician–patient sphere. Mcgill J Med. 2011;13:86.
Wilson IB, Schoen C, Neuman P, Strollo MK, Rogers WH, Chang H, et al. Physician–patient communication about prescription medication nonadherence: a 50-state study of America’s seniors. J Gen Intern Med. 2007;22:6–12.
Tarn DM, Heritage J, Paterniti DA, Hays RD, Kravitz RL, Wenger NS. Prescribing new medications: a taxonomy of physician–patient communication. Commun Med. 2008;5:195–208.
Arora NK. Interacting with cancer patients: the significance of physicians’ communication behavior. Soc Sci Med. 2003;57:791–806.
McKillip RP, Borden BA, Galecki P, Ham SA, Patrick-Miller L, Hall JP, et al. Patient perceptions of care as influenced by a large institutional pharmacogenomic implementation program. Clin Pharmacol Ther 2016;102:106–114.
McKinnon RA, Ward MB, Sorich MJ. A critical analysis of barriers to the clinical implementation of pharmacogenomics. Ther Clin Risk Manag. 2007;3:751–9.
Manolio TA, Chisholm RL, Ozenberger B, Roden DM, Williams MS, Wilson R, et al. Implementing genomic medicine in the clinic: the future is here. Genet Med. 2013;15:258–67.
Haga SB, Mills R, Bosworth H. Striking a balance in communicating pharmacogenetic test results: promoting comprehension and minimizing adverse psychological and behavioral response. Patient Educ Couns. 2014;97:10–15.
Sweet K, Sturm AC, Schmidlen T, Hovick S, Peng J, Manickam K, et al. EMR documentation of physician-patient communication following genomic counseling for actionable complex disease and pharmacogenomic results. Clin Genet. 2017;91:545–56.
Haga SB, LaPointe NM. The potential impact of pharmacogenetic testing on medication adherence. Pharm J. 2013;13:481–3.
Grant RW, Hivert M, Pandiscio JC, Florez JC, Nathan DM, Meigs JB. The clinical application of genetic testing in type 2 diabetes: a patient and physician survey. Diabetologia. 2009;52:2299–305.
Li JH, Joy SV, Haga SB, Orlando LA, Kraus WE, Ginsburg GS, et al. Genetically guided statin therapy on statin perceptions, adherence, and cholesterol lowering: a pilot implementation study in primary care patients. J Pers Med. 2014;4:147–62.
Charland SL, Agatep BC, Herrera V, Schrader B, Frueh FW, Ryvkin M, et al. Providing patients with pharmacogenetic test results affects adherence to statin therapy: results of the Additional KIF6 Risk Offers Better Adherence to Statins (AKROBATS) trial. Pharm J. 2014;14:272–80.
O’Donnell PH, Wadhwa N, Danahey K, Borden BA, Lee SM, Hall JP, et al. Pharmacogenomics-based point-of-care clinical decision support significantly alters drug prescribing. Clin Pharmacol Ther. 2017;102:859–69.
O’Donnell PH, Danahey K, Jacobs M, Wadhwa NR, Yuen S, Bush A, et al. Adoption of a clinical pharmacogenomics implementation program during outpatient care--initial results of the University of Chicago “1,200 Patients Project”. Am J Med Genet C. 2014;166C:68–75.
O’Donnell PH, Bush A, Spitz J, Danahey K, Saner D, Das S, et al. The 1200 patients project: creating a new medical model system for clinical implementation of pharmacogenomics. Clin Pharmacol Ther. 2012;92:446–9.
Hussain S, Kenigsberg BB, Danahey K, Lee YM, Galecki PM, Ratain MJ, et al. Disease-drug database for pharmacogenomic-based prescribing. Clin Pharmacol Ther. 2016;100:179–90.
Danahey K, Borden BA, Furner B, Yukman P, Hussain S, Saner D, et al. Simplifying the use of pharmacogenomics in clinical practice: building the genomic prescribing system. J Biomed Inform. 2017;75:110–21.
Burke W, Emery J. Genetics education for primary-care providers. Nat Rev Genet. 2002;3:561–6.
Sharp RR, Goldlust ME, Eng C. Addressing gaps in physician education using personal genomic testing. Genet Med. 2011;13:750–1.
Unertl KM, Field JR, Price L, Peterson JF. Clinician perspectives on using pharmacogenomics in clinical practice. Per Med. 2015;12:339–47.
Haga SB, Burke W, Ginsburg GS, Mills R, Agans R. Primary care physicians’ knowledge of and experience with pharmacogenetic testing. Clin Genet. 2012;82:388–94.
Martin LR, Williams SL, Haskard KB, Dimatteo MR. The challenge of patient adherence. Ther Clin Risk Manag. 2005;1:189–99.
Kravitz RL, Hays RD, Sherbourne CD, DiMatteo MR, Rogers WH, Ordway L, et al. Recall of recommendations and adherence to advice among patients with chronic medical conditions. Arch Intern Med. 1993;153:1869–78.
Vahdat S, Hamzehgardeshi L, Hessam S, Hamzehgardeshi Z. Patient involvement in health care decision making: a review. Iran Red Crescent Med J. 2014;16:e12454.
Stanek EJ, Sanders CL, Taber KA, Khalid M, Patel A, Verbrugge RR, et al. Adoption of pharmacogenomic testing by US physicians: results of a nationwide survey. Clin Pharmacol Ther. 2012;91:450–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
KD, MJR, and PHO are named as co-inventors on a pending patent application for the Genomic Prescribing System. MJR is a co-inventor holding patents related to pharmacogenetic diagnostics and receives royalties related to UGT1A1 genotyping, although no royalties were received from the genotyping performed in this study. MJR work has been funded by the NIH, The Conquer Cancer Foundation of the American Society for Clinical Oncology, and The William F. O'Connor Foundation. PHO work has been funded by the NIH, The University of Chicago Comprehensive Cancer Center, The University of Chicago Bucksbaum Institute for Clinical Excellence, and the Central Society for Clinical and Translational Research. The remaining authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Borden, B.A., Lee, S.M., Danahey, K. et al. Patient-provider communications about pharmacogenomic results increase patient recall of medication changes. Pharmacogenomics J 19, 528–537 (2019). https://doi.org/10.1038/s41397-019-0076-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41397-019-0076-2
This article is cited by
-
Anti-tumoral activity of Allium roseum compounds on breast cancer cells MCF7 and MDA-MB231
Advances in Traditional Medicine (2023)
-
Underrepresented patient views and perceptions of personalized medication treatment through pharmacogenomics
npj Genomic Medicine (2021)