Abstract
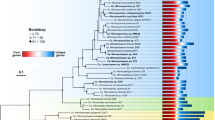
After decades studying the microbial “deep biosphere” in subseafloor oceanic crust, the growth and life strategies in this anoxic, low energy habitat remain poorly described. Using both single cell genomics and metagenomics, we reveal the life strategies of two distinct lineages of uncultivated Aminicenantia bacteria from the basaltic subseafloor oceanic crust of the eastern flank of the Juan de Fuca Ridge. Both lineages appear adapted to scavenge organic carbon, as each have genetic potential to catabolize amino acids and fatty acids, aligning with previous Aminicenantia reports. Given the organic carbon limitation in this habitat, seawater recharge and necromass may be important carbon sources for heterotrophic microorganisms inhabiting the ocean crust. Both lineages generate ATP via several mechanisms including substrate-level phosphorylation, anaerobic respiration, and electron bifurcation driving an Rnf ion translocation membrane complex. Genomic comparisons suggest these Aminicenantia transfer electrons extracellularly, perhaps to iron or sulfur oxides consistent with mineralogy of this site. One lineage, called JdFR-78, has small genomes that are basal to the Aminicenantia class and potentially use “primordial” siroheme biosynthetic intermediates for heme synthesis, suggesting this lineage retain characteristics of early evolved life. Lineage JdFR-78 contains CRISPR-Cas defenses to evade viruses, while other lineages contain prophage that may help prevent super-infection or no detectable viral defenses. Overall, genomic evidence points to Aminicenantia being well adapted to oceanic crust environments by taking advantage of simple organic molecules and extracellular electron transport.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All SAG data for this project can be found on NCBI under BioProject ID PRJNA842252 under accession numbers: JAMZRZ000000000 (JDF1 composite genome); JAMZSA000000000 (AH-873-B07); JAMZSB000000000 (AC-708-M15); JAMZSC000000000 (AC-708-I09); JAMZSD000000000 (AC-335-O07); JAMZSE000000000 (AC-335-L06); JAMZSF000000000 (AC-335-K20); JAMZSG000000000 (AC-335-G13); JAMZSH000000000 (AC-335-B20); JAMZSI000000000 (AC-335-A11); JAMZSJ000000000 (AC-334-K16); JAMZSK000000000 (AC-334-E05). All MAG data for this project can be found on IMG.
References
Bar-On YM, Phillips R, Milo R. The biomass distribution on Earth. Proc Natl Acad Sci USA. 2018;115:6506–11. https://doi.org/10.1073/pnas.1711842115.
Orcutt BN, D’angelo T, Jungbluth SP, Huber JA, Sylvan JB. Microbial life in oceanic crust. 2020. https://doi.org/10.31219/osf.io/2wxe6.
Edwards KJ, Fisher AT, Wheat CG. The deep subsurface biosphere in igneous ocean crust: frontier habitats for microbiological exploration. Front Microbiol. 2012;3:8. https://doi.org/10.3389/fmicb.2012.00008.
Orcutt BN, Edwards KJ. Life in the ocean crust: lessons from subseafloor laboratories. Dev Mar Geol. 2014;7:175–96. https://doi.org/10.1016/B978-0-444-62617-2.00007-4.
Cowen JP, Giovannoni SJ, Kenig F, Johnson HP, Butterfield D, Rappé MS, et al. Fluids from aging ocean crust that support microbial life. Science. 2003;299:120–3. https://doi.org/10.1126/science.1075653.
Jungbluth SP, Bowers RM, Lin H-T, Cowen JP, Rappé MS. Novel microbial assemblages inhabiting crustal fluids within mid-ocean ridge flank subsurface basalt. ISME J. 2016;10:2033–47. https://doi.org/10.1038/ismej.2015.248.
Nigro OD, Jungbluth SP, Lin H, Hsieh C, Miranda JA, Schvarcz CR, et al. Viruses in the oceanic basement. mBio. 2017;8. https://doi.org/10.1128/mBio.02129-16.
Carr SA, Jungbluth SP, Eloe-Fadrosh EA, Stepanauskas R, Woyke T, Rappé MS, et al. Carboxydotrophy potential of uncultivated hydrothermarchaeota from the subseafloor crustal biosphere. ISME J. 2019;13:1457–68. https://doi.org/10.1038/s41396-019-0352-9.
Ivarsson M, Bengtson S, Neubeck A. The igneous oceanic crust—Earth’s largest fungal habitat? Fungal Ecol. 2016;20:249–55. https://doi.org/10.1016/j.funeco.2016.01.009.
Smith AR, Mueller R, Fisk MR, Colwell FS. Ancient metabolisms of a thermophilic subseafloor bacterium. Front Microbiol. 2021;12:764631. https://doi.org/10.3389/fmicb.2021.764631.
Smith AR, Kieft B, Mueller R, Fisk MR, Mason OU, Popa R, et al. Carbon fixation and energy metabolisms of a subseafloor olivine biofilm. ISME J. 2019;13:1737–49. https://doi.org/10.1038/s41396-019-0385-0.
Fisher AT, Davis EE, Hutnak M, Spiess V, Zühlsdorff L, Cherkaoui A, et al. Hydrothermal recharge and discharge across 50 km guided by seamounts on a young ridge flank. Nature. 2003;421:618–21. https://doi.org/10.1038/nature01352.
Orcutt BN, Bach W, Becker K, Fisher AT, Hentscher M, Toner BM, et al. Colonization of subsurface microbial observatories deployed in young ocean crust. ISME J. 2011;5:692–703. https://doi.org/10.1038/ismej.2010.157.
Fisher AT, Tsuji T, Petronotis K, Wheat CG, Becker K, Clark JF, et al. IODP expedition 327 and Atlantis expedition AT 18-07: observatories and experiments on the eastern flank of the Juan de Fuca Ridge. Sci Drill. 2012. https://doi.org/10.2204/iodp.sd.13.01.2011.
Smith AR, Fisk MR, Thurber AR, Flores GE, Mason OU, Popa R, et al. Deep crustal communities of the Juan de Fuca Ridge are governed by mineralogy. Geomicrobiol J. 2017;34:147–56. https://doi.org/10.1080/01490451.2016.1155001.
Jungbluth SP, Amend JP, Rappé MS. Metagenome sequencing and 98 microbial genomes from Juan de Fuca Ridge flank subsurface fluids. Sci Data. 2017;4:170037. https://doi.org/10.1038/sdata.2017.37.
Ramírez GA, Garber AI, Lecoeuvre A, D’Angelo T, Wheat CG, Orcutt BN. Ecology of subseafloor crustal biofilms. Front Microbiol. 2019;10:1983. https://doi.org/10.3389/fmicb.2019.01983.
Robador A, LaRowe DE, Jungbluth SP, Lin H-T, Rappé MS, Nealson KH, et al. Nanocalorimetric characterization of microbial activity in deep subsurface oceanic crustal fluids. Front Microbiol. 2016;7:454. https://doi.org/10.3389/fmicb.2016.00454.
LaRowe DE, Koch BP, Robador A, Witt M, Ksionzek K, Amend JP. Identification of organic compounds in ocean basement fluids. Org Geochem. 2017;113:124–7. https://doi.org/10.1016/j.orggeochem.2017.07.017.
Lu GS, LaRowe DE, Amend JP. Bioenergetic potentials in terrestrial, shallow-sea and deep-sea hydrothermal systems. Chem Geol. 2021;583:120449. https://doi.org/10.1016/j.chemgeo.2021.120449.
Jungbluth SP, Grote J, Lin HT, Cowen JP, Rappé MS. Microbial diversity within basement fluids of the sediment-buried Juan de Fuca Ridge flank. ISME J. 2013;7:161–72. https://doi.org/10.1038/ismej.2012.73.
Castelle CJ, Banfield JF. Major new microbial groups expand diversity and alter our understanding of the tree of life. Cell. 2018;172:1181–97. https://doi.org/10.1016/j.cell.2018.02.016.
Farag IF, Davis JP, Youssef NH, Elshahed MS. Global patterns of abundance, diversity and community structure of the Aminicenantes (Candidate Phylum OP8). PLoS ONE. 2014;9:e92139. https://doi.org/10.1371/journal.pone.0092139.
Rinke C, Schwientek P, Sczyrba A, Ivanova NN, Anderson IJ, Cheng J-F, et al. Insights into the phylogeny and coding potential of microbial dark matter. Nature. 2013;499:431–7. https://doi.org/10.1038/nature12352.
Parks DH, Chuvochina M, Rinke C, Mussig AJ, Chaumeil P-A, Hugenholtz P. GTDB: an ongoing census of bacterial and archaeal diversity through a phylogenetically consistent, rank normalized and complete genome-based taxonomy. Nucleic Acids Res. 2022;50:D785–94. https://doi.org/10.1093/nar/gkab776.
Oren A, Garrity GM. Valid publication of the names of forty-two phyla of prokaryotes. Int J Syst Evol Microbiol. 2021;71. https://doi.org/10.1099/ijsem.0.005056.
Hugenholtz P, Pitulle C, Hershberger KL, Pace NR. Novel division level bacterial diversity in a Yellowstone hot spring. J Bacteriol. 1998;180:366–76. https://doi.org/10.1128/JB.180.2.366-376.1998.
Kadnikov VV, Mardanov AV, Beletsky AV, Karnachuk OV, Ravin NV. Genome of the candidate phylum Aminicenantes bacterium from a deep subsurface thermal aquifer revealed its fermentative saccharolytic lifestyle. Extremophiles. 2019;23:189–200. https://doi.org/10.1007/s00792-018-01073-5.
Begmatov S, Savvichev AS, Kadnikov VV, Beletsky AV, Rusanov II, Klyuvitkin AA, et al. Microbial communities involved in methane, sulfur, and nitrogen cycling in the sediments of the barents sea. Microorganisms. 2021;9:2362. https://doi.org/10.3390/microorganisms9112362.
Semler AC, Fortney JL, Fulweiler RW, Dekas AE. Cold seeps on the passive Northern U.S. Atlantic Margin host globally representative members of the seep microbiome with locally dominant strains of archaea. Appl Environ Microbiol. 2022;88:0046822. https://doi.org/10.1128/aem.00468-22.
Robbins SJ, Evans PN, Parks DH, Golding SD, Tyson GW. Genome-centric analysis of microbial populations enriched by hydraulic fracture fluid additives in a coal bed methane production well. Front Microbiol. 2016;7:731. https://doi.org/10.3389/fmicb.2016.00731.
Lin HT, Cowen JP, Olson EJ, Amend JP, Lilley MD. Inorganic chemistry, gas compositions and dissolved organic carbon in fluids from sedimented young basaltic crust on the Juan de Fuca Ridge flanks. Geochim Cosmochim Acta. 2012;85:213–27. https://doi.org/10.1016/j.gca.2012.02.017.
Fisher AT, Wheat CG, Becker K, Cowen J, Orcutt B, Hulme S, et al. Design, deployment, and status of borehole observatory systems used for single-hole and cross-hole experiments, IODP Expedition 327, eastern flank of Juan de Fuca Ridge. Proc Integr Ocean Drill Progr. 2011. https://doi.org/10.2204/iodp.proc.327.107.2011.
Fisher AT, Urabe T, Klaus A,and the Expedition 301 Scientists. Expedition 301 summary. Proc Integr Ocean Drill Progr. 2005. https://doi.org/10.2204/iodp.proc.301.101.2005.
Wheat CG, Hulme SM, Fisher AT, Orcutt BN, Becker K. Seawater recharge into oceanic crust: IODP Exp 327 Site U1363 Grizzly Bare outcrop. Geochem Geophys Geosyst. 2013;14:1957–72. https://doi.org/10.1002/ggge.20131.
Wheat CG, Jannasch HW, Kastner M, Hulme S, Cowen J, Edwards KJ, et al. Fluid sampling from oceanic borehole observatories: design and methods for CORK activities (1990-2010). Proc Integr Ocean Drill Progr. 2011. https://doi.org/10.2204/iodp.proc.327.109.2011.
Clum A, Huntemann M, Bushnell B, Foster B, Foster B, Roux S, et al. DOE JGI metagenome workflow. mSystems. 2021;6. https://doi.org/10.1128/mSystems.00804-20.
Stepanauskas R, Fergusson EA, Brown J, Poulton NJ, Tupper B, Labonté JM, et al. Improved genome recovery and integrated cell-size analyses of individual uncultured microbial cells and viral particles. Nat Commun. 2017;8:84. https://doi.org/10.1038/s41467-017-00128-z.
Kanehisa M, Sato Y. KEGG Mapper for inferring cellular functions from protein sequences. Protein Sci. 2020;29:28–35. https://doi.org/10.1002/pro.3711.
Aramaki T, Blanc-Mathieu R, Endo H, Ohkubo K, Kanehisa M, Goto S, et al. KofamKOALA: KEGG Ortholog assignment based on profile HMM and adaptive score threshold. Bioinformatics. 2020;36:2251–2. https://doi.org/10.1093/bioinformatics/btz859.
Graham ED, Heidelberg JF, Tully BJ. Potential for primary productivity in a globally-distributed bacterial phototroph. ISME J. 2018;12:1861–6. https://doi.org/10.1038/s41396-018-0091-3.
Yin Y, Mao X, Yang J, Chen X, Mao F, Xu Y. dbCAN: a web resource for automated carbohydrate-active enzyme annotation. Nucleic Acids Res. 2012;40:W445–51. https://doi.org/10.1093/nar/gks479.
de Castro E, Sigrist CJA, Gattiker A, Bulliard V, Langendijk-Genevaux PS, Gasteiger E, et al. ScanProsite: detection of PROSITE signature matches and ProRule-associated functional and structural residues in proteins. Nucleic Acids Res. 2006;34:W362–5. https://doi.org/10.1093/nar/gkl124.
Krogh A, Larsson B, Von Heijne G, Sonnhammer E. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 2001;305:567–80. https://doi.org/10.1006/jmbi.2000.4315.
Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–7. https://doi.org/10.1093/nar/gkh340.
Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–3. https://doi.org/10.1093/bioinformatics/btu033.
Roux S, Enault F, Hurwitz BL, Sullivan MB. VirSorter: mining viral signal from microbial genomic data. PeerJ. 2015;3:e985. https://doi.org/10.7717/peerj.985.
Bland C, Ramsey TL, Sabree F, Lowe M, Brown K, Kyrpides NC, et al. CRISPR Recognition Tool (CRT): a tool for automatic detection of clustered regularly interspaced palindromic repeats. BMC Bioinform. 2007;8:209. https://doi.org/10.1186/1471-2105-8-209.
Padilha VA, Alkhnbashi OS, Shah SA, de Carvalho ACPLF, Backofen R. CRISPRcasIdentifier: machine learning for accurate identification and classification of CRISPR-Cas systems. Gigascience. 2020;9. https://doi.org/10.1093/gigascience/giaa062.
Campbell JW, Morgan-Kiss RM, Cronan JE. A new Escherichia coli metabolic competency: growth on fatty acids by a novel anaerobic b-oxidation pathway. Mol Microbiol. 2003;47:793–805. https://doi.org/10.1046/j.1365-2958.2003.03341.x.
Sharon I, Kertesz M, Hug LA, Pushkarev D, Blauwkamp TA, Castelle CJ, et al. Accurate, multi-kb reads resolve complex populations and detect rare microorganisms. Genome Res. 2015;25:534–43. https://doi.org/10.1101/gr.183012.114.
Lin HT, Amend JP, LaRowe DE, Bingham JP, Cowen JP. Dissolved amino acids in oceanic basaltic basement fluids. Geochim Cosmochim Acta. 2015;164:175–90. https://doi.org/10.1016/j.gca.2015.04.044.
Lomstein BA, Langerhuus AT, D’Hondt S, Jørgensen BB, Spivack AJ. Endospore abundance, microbial growth and necromass turnover in deep sub-seafloor sediment. Nature. 2012;484:101–4. https://doi.org/10.1038/nature10905.
Robador A, Jungbluth SP, LaRowe DE, Bowers RM, Rappé MS, Amend JP, et al. Activity and phylogenetic diversity of sulfate-reducing microorganisms in low-temperature subsurface fluids within the upper oceanic crust. Front Microbiol. 2015;5:748. https://doi.org/10.3389/fmicb.2014.00748.
Boettger J, Lin HT, Cowen JP, Hentscher M, Amend JP. Energy yields from chemolithotrophic metabolisms in igneous basement of the Juan de Fuca ridge flank system. Chem Geol. 2013;337-338:11–19. https://doi.org/10.1016/j.chemgeo.2012.10.053.
Lang SQ, Butterfield DA, Lilley MD, Paul Johnson H, Hedges JI. Dissolved organic carbon in ridge-axis and ridge-flank hydrothermal systems. Geochim Cosmochim Acta. 2006;70:3830–42. https://doi.org/10.1016/j.gca.2006.04.031.
Haveman SA, Holmes DE, Ding YHR, Ward JE, DiDonato RJ, Lovley DR. c-type cytochromes in Pelobacter carbinolicus. Appl Environ Microbiol. 2006;72:6980–5. https://doi.org/10.1128/AEM.01128-06.
Izumi H, Nunoura T, Miyazaki M, Mino S, Toki T, Takai K, et al. Thermotomaculum hydrothermale gen. nov., sp. nov., a novel heterotrophic thermophile within the phylum Acidobacteria from a deep-sea hydrothermal vent chimney in the Southern Okinawa Trough. Extremophiles. 2012;16:245–53. https://doi.org/10.1007/s00792-011-0425-9.
Fisher AT, Tsujii T, Petronotis K,Expedition 327 Scientists. Site U1362. Proc Integr Ocean Drill Progr. 2011. https://doi.org/10.2204/iodp.proc.327.103.2011.
Vital M, Howe AC, Tiedje JM. Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. mBio. 2014;5:00889. https://doi.org/10.1128/mBio.00889-14.
Chowdhury NP, Mowafy AM, Demmer JK, Upadhyay V, Koelzer S, Jayamani E, et al. Studies on the mechanism of electron bifurcation catalyzed by electron transferring flavoprotein (Etf) and butyryl-CoA dehydrogenase (Bcd) of Acidaminococcus fermentans. J Biol Chem. 2014;289:5145–57. https://doi.org/10.1074/jbc.M113.521013.
Poudel S, Dunham EC, Lindsay MR, Amenabar MJ, Fones EM, Colman DR, et al. Origin and evolution of flavin-based electron bifurcating enzymes. Front Microbiol. 2018;9:1762. https://doi.org/10.3389/fmicb.2018.01762.
Charon M-H, Volbeda A, Chabriere E, Pieulle L, Fontecilla-Camps JC. Structure and electron transfer mechanism of pyruvate:ferredoxin oxidoreductase. Curr Opin Struct Biol. 1999;9:663–9. https://doi.org/10.1016/S0959-440X(99)00027-5.
Kuhns M, Trifunović D, Huber H, Müller V. The Rnf complex is a Na+ coupled respiratory enzyme in a fermenting bacterium, Thermotoga maritima. Commun Biol. 2020;3:431. https://doi.org/10.1038/s42003-020-01158-y.
Stojanowic A, Mander GJ, Duin EC, Hedderich R. Physiological role of the F420-non-reducing hydrogenase (MvH) from Methanothermobacter marburgensis. Arch Microbiol. 2003;180:194–203. https://doi.org/10.1007/s00203-003-0577-9.
Søndergaard D, Pedersen CNS, Greening C. HydDB: a web tool for hydrogenase classification and analysis. Sci Rep. 2016;6:34212. https://doi.org/10.1038/srep34212.
Yan Z, Wang M, Ferry JG. A ferredoxin- and F420H2-dependent, electron-bifurcating, heterodisulfide reductase with homologs in the domains Bacteria and Archaea. mBio. 2017;8. https://doi.org/10.1128/mBio.02285-16.
Lin H-T, Cowen JP, Olson EJ, Lilley MD, Jungbluth SP, Wilson ST, et al. Dissolved hydrogen and methane in the oceanic basaltic biosphere. Earth Planet Sci Lett. 2014;405:62–73. https://doi.org/10.1016/j.epsl.2014.07.037.
Gencic S, Grahame DA. Diverse energy-conserving pathways in Clostridium difficile: growth in the absence of amino acid stickland acceptors and the role of the Wood-Ljungdahl pathway. J Bacteriol. 2020;202. https://doi.org/10.1128/JB.00233-20.
Ragsdale SW, Pierce E. Acetogenesis and the Wood-Ljungdahl pathway of CO2 fixation. Biochim Biophys Acta. 2008;1784:1873–98. https://doi.org/10.1016/j.bbapap.2008.08.012.
Wheat CG, Jannasch HW, Fisher AT, Becker K, Sharkey J, Hulme S. Subseafloor seawater-basalt-microbe reactions: continuous sampling of borehole fluids in a ridge flank environment. Geochem Geophys Geosyst. 2010;11. https://doi.org/10.1029/2010GC003057.
Buckel W, Thauer RK. Flavin-based electron bifurcation, a new mechanism of biological energy coupling. Chem Rev. 2018;118:3862–86. https://doi.org/10.1021/acs.chemrev.7b00707.
Mulkidjanian AY, Galperin MY, Makarova KS, Wolf YI, Koonin EV. Evolutionary primacy of sodium bioenergetics. Biol Direct. 2008;3:13. https://doi.org/10.1186/1745-6150-3-13.
Jungbluth SP, Glavina del Rio T, Tringe SG, Stepanauskas R, Rappé MS. Genomic comparisons of a bacterial lineage that inhabits both marine and terrestrial deep subsurface systems. PeerJ. 2017;5:e3134. https://doi.org/10.7717/peerj.3134.
Fincker M, Huber JA, Orphan VJ, Rappé MS, Teske A, Spormann AM. Metabolic strategies of marine subseafloor Chloroflexi inferred from genome reconstructions. Environ Microbiol. 2020;22:3188–204. https://doi.org/10.1111/1462-2920.15061.
Bach W, Edwards KJ. Iron and sulfide oxidation within the basaltic ocean crust: implications for chemolithoautotrophic microbial biomass production. Geochim Cosmochim Acta. 2003;67:3871–87. https://doi.org/10.1016/S0016-7037(03)00304-1.
Kauffman KM, Chang WK, Brown JM, Hussain FA, Yang J, Polz MF, et al. Resolving the structure of phage–bacteria interactions in the context of natural diversity. Nat Commun. 2022;13:372. https://doi.org/10.1038/s41467-021-27583-z.
Anderson RE, Brazelton WJ, Baross JA. Using CRISPRs as ametagenomic tool to identify microbial hosts of a diffuse flow hydrothermal vent viral assemblage. FEMS Microbiol Ecol. 2011;77:120–33. https://doi.org/10.1111/j.1574-6941.2011.01090.x.
Howard-Varona C, Hargreaves KR, Abedon ST, Sullivan MB. Lysogeny in nature: mechanisms, impact and ecology of temperate phages. ISME J. 2017;11:1511–20. https://doi.org/10.1038/ismej.2017.16.
Bondy-Denomy J, Qian J, Westra ER, Buckling A, Guttman DS, Davidson AR, et al. Prophages mediate defense against phage infection through diverse mechanisms. ISME J. 2016;10:2854–66. https://doi.org/10.1038/ismej.2016.79.
Deutschmann J, Gramberg T. SAMHD1… And viral ways around it. Viruses. 2021;13:395. https://doi.org/10.3390/v13030395.
Gronow S, Brade H. Lipopolysaccharide biosynthesis: which steps do bacteria need to survive. J Endotoxin Res. 2001;7:3–23. https://doi.org/10.1179/096805101101532468.
Weinberger AD, Wolf YI, Lobkovsky AE, Gilmore MS, Koonin EV. Viral diversity threshold for adaptive immunity in prokaryotes. mBio. 2012;3:00456–12. https://doi.org/10.1128/mBio.00456-12.
McGinn J, Marraffini LA. Molecular mechanisms of CRISPR–Cas spacer acquisition. Nat Rev Microbiol. 2019;17:7–12. https://doi.org/10.1038/s41579-018-0071-7.
Pachiadaki MG, Brown JM, Brown J, Bezuidt O, Berube PM, Biller SJ, et al. Charting the complexity of the marine microbiome through single-cell genomics. Cell. 2019;179:1623–35.e11. https://doi.org/10.1016/j.cell.2019.11.017.
Makarova KS, Haft DH, Barrangou R, Brouns SJJ, Charpentier E, Horvath P, et al. Evolution and classification of the CRISPR-Cas systems. Nat Rev Microbiol. 2011;9:467–77. https://doi.org/10.1038/nrmicro2577.
Hale CR, Cocozaki A, Li H, Terns RM, Terns MP. Target RNA capture and cleavage by the Cmr type III-B CRISPR–Cas effector complex. Genes Dev. 2014;28:2432–43. https://doi.org/10.1101/gad.250712.114.
Han W, Stella S, Zhang Y, Guo T, Sulek K, Peng-Lundgren L, et al. A Type III-B Cmr effector complex catalyzes the synthesis of cyclic oligoadenylate second messengers by cooperative substrate binding. Nucleic Acids Res. 2018;46:10319–30. https://doi.org/10.1093/nar/gky844.
Staals RHJ, Agari Y, Maki-Yonekura S, Zhu Y, Taylor DW, van Duijn E, et al. Structure and activity of the RNA-targeting Type III-B CRISPR-Cas complex of Thermus thermophilus. Mol Cell. 2013;52:135–45. https://doi.org/10.1016/j.molcel.2013.09.013.
Wang L, Mo CY, Wasserman MR, Rostøl JT, Marraffini LA, Liu S. Dynamics of Cas10 govern discrimination between self and non-self in Type III CRISPR-Cas immunity. Mol Cell. 2019;73:278–90.e4. https://doi.org/10.1016/j.molcel.2018.11.008.
Koonin EV, Wolf YI. Evolution of the CRISPR-Cas adaptive immunity systems in prokaryotes: models and observations on virus–host coevolution. Mol Biosyst. 2015;11:20–7. https://doi.org/10.1039/C4MB00438H.
Dailey HA, Dailey TA, Gerdes S, Jahn D, Jahn M, O’Brian MR, et al. Prokaryotic heme biosynthesis: multiple pathways to a common essential product. Microbiol Mol Biol Rev. 2017;81. https://doi.org/10.1128/mmbr.00048-16.
Murphy MJ, Siegel LM, Tove SR, Kamin H. Siroheme: a new prosthetic group participating in six-electron reduction reactions catalyzed by both sulfite and nitrite reductases. Proc Natl Acad Sci USA. 1974;71:612–6. https://doi.org/10.1073/pnas.71.3.612.
Xavier JC, Gerhards RE, Wimmer JLE, Brueckner J, Tria FDK, Martin WF. The metabolic network of the last bacterial common ancestor. Commun Biol. 2021;4:413. https://doi.org/10.1038/s42003-021-01918-4.
Giovannoni SJ, Thrash JC, Temperton B. Implications of streamlining theory for microbial ecology. ISME J. 2014;8:1553–65. https://doi.org/10.1038/ismej.2014.60.
Hubert C, Loy A, Nickel M, Arnosti C, Baranyi C, Brüchert V, et al. A constant flux of diverse thermophilic bacteria into the cold Arctic seabed. Science. 2009;325:1541–4. https://doi.org/10.1126/science.1174012.
Müller AL, de Rezende JR, Hubert CRJ, Kjeldsen KU, Lagkouvardos I, Berry D, et al. Endospores of thermophilic bacteria as tracers of microbial dispersal by ocean currents. ISME J. 2014;8:1153–65. https://doi.org/10.1038/ismej.2013.225.
Walsh EA, Kirkpatrick JB, Rutherford SD, Smith DC, Sogin M, D’Hondt S. Bacterial diversity and community composition from seasurface to subseafloor. ISME J. 2016;10:979–89. https://doi.org/10.1038/ismej.2015.175.
Jørgensen SL, Zhao R. Microbial inventory of deeply buried oceanic crust from a young ridge flank. Front Microbiol. 2016;7:820. https://doi.org/10.3389/fmicb.2016.00820.
Labonté JM, Lever MA, Edwards KJ, Orcutt BN. Influence of igneous basement on deep sediment microbial diversity on the Eastern Juan de Fuca Ridge flank. Front Microbiol. 2017;8:1434. https://doi.org/10.3389/fmicb.2017.01434.
Acknowledgements
The authors thank the science parties and crews of expeditions AT18-07, AT26-18 and AT42-11 for their support to collect the samples used in this study. We also thank Jennifer Gladstone, Anna Chen, Melody Lindsay and Julia McGonigle for discussions that helped shape the direction of this study, the staff of the Single Cell Genomics Center at the Bigelow Laboratory for Ocean Science and the Joint Genome Institute (JGI) for all sequencing, and Ruth Booker and Orion Thomas for extensive proof-reading. Samples from 2019 were collected with the consent of the Government of Canada, as reviewed by Global Affairs Canada.
Funding
Funding for the expeditions was provided by the U.S. National Science Foundation (awards OCE-1260723 to MSR, OCE-1260548 to C. Geoff Wheat, and OCE-1737017 to BNO). Funding for analyses was provided in part from the NSF (award OCE-173017 to BNO, OCE-1851582 to MSR, and OIA-1826734 to RS and BNO), from the NASA Exobiology program (80NSSC19K0466 to BNO), from the Center for Dark Energy Biosphere Investigation (C-DEBI; subaward from OCE-0939654 to BNO), and from the Rodney White Fellowship provided by Bigelow Laboratory for Ocean Sciences (to AEB and AAB). The work conducted by the US Department of Energy JGI, a US Department of Energy Office of Science User Facility, is supported under contract no. DE-AC02-05CH11231. This is HIMB contribution number 1933 and SOEST contribution number 11682.
Author information
Authors and Affiliations
Contributions
AEB, BNO, JMB, and TD conceived the project and designed the analyses. BNO, MSR, and RS secured funding. AEB, TD, AAB, JMB performed the analyses. All authors interpreted the results and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Booker, A.E., D’Angelo, T., Adams-Beyea, A. et al. Life strategies for Aminicenantia in subseafloor oceanic crust. ISME J 17, 1406–1415 (2023). https://doi.org/10.1038/s41396-023-01454-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41396-023-01454-5