Abstract
Introduction
Post-traumatic syringomyelia is an uncommon complication after traumatic spinal cord injury. This case study details our decision-making and surgical approach for a patient with symptomatic post-traumatic syringomyelia after sustaining a gunshot wound.
Case presentation
A 24-year-old man with past medical history of distant American Spinal Injury Association Impairment Grade B spinal cord injury due to ballistic injury developed delayed post-traumatic syringomyelia, resulting in unilateral sensory loss and left upper extremity weakness. CT and MR imaging revealed a syrinx spanning his cervical and thoracic spine causing significant spinal cord compression. To relieve achieve decompression and restore CSF flow dynamics, we performed a bony extradural decompression, bullet fragment extraction, spinal cord untethering, and midline myelotomy. Postoperatively, the patient demonstrated clinical and radiographical improvement.
Discussion
Post-traumatic syringomyelia is potentially morbid sequalae of spinal cord injuries. Suspicion for post-traumatic syringomyelia should be maintained in patients with delayed, progressive neurologic deficits. In this setting, surgical intervention may require extradural and intradural procedures to mitigate neural compression along the dilated central canal by the syrinx.
Similar content being viewed by others
Introduction
Post-traumatic syringomyelia (PTS) is a rare and potentially morbid complication seen after spinal cord injury (SCI). PTS can manifest with neurological findings below the level of the original SCI. PTS should be suspected in patients with history of SCI and progressive neurologic symptoms. Diagnosis is confirmed with imaging of the spinal cord. To date, there is no consensus on the optimal surgical approach to definitively treat this condition. In this report, we present the case of a patient who developed PTS 14 years after a penetrating ballistic SCI, detail our surgical approach, and elaborate on our experience in the context of the literature describing surgical management of PTS.
Case presentation
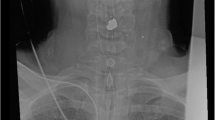
In 2007, a left-handed adolescent male suffered multiple gunshot wounds to the neck and left chest. On initial presentation, he had emergent thoracostomy tube placement followed by intubation for airway protection. Computed tomography (CT) of the cervicothoracic spine demonstrated multi-level penetrating injury, with ballistic fragments abutting the left posterior elements and vertebral body of C7 and a complex, comminuted T4 fracture with cord transection at this level, resulting in American Spinal Injury Association Impairment (ASIA) Grade B injury (Fig. 1A, B.1-2). Operative management of the SCI was deferred.
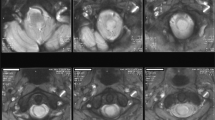
More than 14 years after this initial injury, the now 24-year-old patient was admitted due to inability to care for himself and failure to thrive. He had no other contributory medical, family, and psycho-social history. However, after additional questioning, this inability to care for himself was due to progressive left-handed clumsiness secondary to numbness and tingling. He also endorsed worsening neuropathic pain, more pronounced in his left arm. Formal neurologic examination revealed asymmetric 4+ strength in left hand grip and intrinsic muscles. Assessment of light touch and pain sensation was carried out using a cotton-wrapped stick and the sharp end of a snapped cotton-wrapped stick, respectively. Light touch and pain stimuli were applied 5–10 times per body region. The patient’s sensory exam was notable for decreased sensation of the left upper extremity, as he was only able to correctly identify 10–20% of light touch or sharp stimuli. Chronic paraplegia with significant bilateral lower extremity contractures and long-standing T4 sensory level were unchanged from baseline. Given these new neurologic deficits, CT myelogram was obtained which revealed extensive syrinx with associated expansion of the spinal cord, extending from the craniocervical junction to T4, a new finding from prior CT myelogram, which was obtained after the initial injury in 2007 (Fig. 2). To aid in surgical decision-making, magnetic resonance imaging (MRI) was obtained. MRI revealed tethering of the spinal cord at the T4 level, resulting in an ascending expansile syringomyelia (Fig. 3A, B).
Remote (A, B, 5/2007) and recent (C, D, 6/2021) computed topography myelogram studies. A Sagittal image, demonstrated bullet fragments at the anterior C6-7 vertebral bodies and a burst fracture of the T4 vertebral body with ballistic fragments anterior to the vertebral body and within the spinal canal. Axial imaging shows the thecal sac and spinal cord are displaced posteriorly at C6 (B.1) and left posterolaterally at T4 (B.2). C Sagittal image, showing longitudinally extensive syringomyelia with associated spinal cord expansion extending from the craniocervical junction to the level of C7 demonstrated by axial images with cross-sectional diameter of 1.4 cm (D.1) and 1.2 cm (D.2).
A Preoperative workup, T2 sagittal image, demonstrated large cervicothoracic syrinx with septations with a maximal cross-sectional diameter of 1.1 × 1.2 cm at C6-7 on axial MRI (B) (1.5T, 3 mm slice thickness). C Postoperative T4 corpectomy and bullet removal, T2 sagittal image showed moderately decompressed syrinx. D Axial image at C6-7, showed a decrease in cross-sectional diameter of 0.7 × 0.9 cm.
Operative procedure
Given this symptomatic PTS, patient was consented and taken for detethering of the spinal cord to re-establish normal cerebrospinal fluid (CSF) dynamics. To visualize the ballistic fragment responsible for spinal cord deviation, laminectomies were performed at T3-5 with complete right T3 and T4 facetectomy. Due the bullet’s firm lodging into the T4 vertebral body, removal required fragmentation of the bullet with a high-speed drill using the surgical microscope. Furthermore, partial corpectomies at T4 and T5 were employed to achieve circumferential extradural spinal cord decompression.
Next, the dura was opened in a right paramedian fashion and revealed a thickened, milky arachnoid layer. No spontaneous CSF flow was observed. With microscopic assistance, the arachnoid adhesions were taken down at the level of T3 and T4 on the right side, with return of CSF flow. Intraoperative ultrasound was used to visualize the syrinx and assess for septations. Ultrasound revealed persistent expansion of the cord rostral to the level of the detethering. These imaging findings were used to perform a 5-mm midline myelotomy at the largest loculated region of the syrinx. This resulted in immediate egress of CSF from the spinal cord and sonographic evidence of syrinx improvement. An expansive duraplasty was performed and dorsal arch bars were fashioned to recapitulate the lamina. Closure was performed in a standard layered fashion with one subfascial drain maintained to gravity.
Postoperative course
In the immediate postoperative setting, the patient reported subjective improvement in left upper extremity numbness and slight improvement in left hand grip and intrinsic strength. Two weeks postoperatively, MRI confirmed decompression of the syrinx (Figs. 3C, D and 4) with a notable decrease in cross-sectional diameter at the level of T2 (Fig. 3, 1.1 × 1.2 cm to 0.7 × 0.9 cm). The patient was doing well at 1-month follow-up, evidenced by 5/5 left hand grip and 5/5 intrinsic muscle strength (compared to 4+/5 preoperatively) and left upper extremity sensation that was only 40–50% decreased compared to right upper extremity (compared to 80–90% decrease preoperatively). The patient followed up in clinic at 20-weeks after surgery with continued improvement in motor and sensory function.
Discussion
In 2020, the estimated number of people living with SCI in the US was ~294,000 [1]. PTS is a rare complication seen days [2] to years [3] after SCI that results in the development of a CSF-filled cavity within the spinal cord. The pathogenesis of PTS remains unproven, but is likely attributable to subarachnoid scarring secondary to the primary SCI that perturbs perivascular fluid mechanics of CSF and blood [4]. Before the turn of the twentieth century, PTS had been described after SCI sustained from ballistic trauma [5] and high falls [2, 6]. At present, motor vehicle accidents present the most common mechanism of SCI, followed by falls, acts of violence (primarily gunshot wounds), and sports/recreation activities [7]. Among those with SCI, 1–7% will develop symptomatic syringomyelia [8, 9]. Symptom presentation is varied, but can include neuropathic pain [4, 10,11,12], sensory loss [4, 12,13,14], motor impairment [4, 10,11,12] and/or autonomic instability [4, 15, 16]. Modern neuroimaging techniques have aided diagnosis of PTS. T2-weighted MR imaging is the most sensitive technique and preferred for diagnosis, morphological characterization and serial monitoring [17, 18]. In cases where MRI is unavailable or contraindicated, CT with contrast myelography has demonstrated clinical utility, but 10–50% of syringes remain undetected [17].
Symptomatology dictates management. In cases without severe or progressive neuropathic pain and motor weakness, conservative management has demonstrated durability of symptom stabilization or even improvement with rehabilitation [16, 19,20,21]. However, in the setting of neurological deterioration and/or severe pain, surgical intervention becomes necessary. The goal of surgery is to achieve internal decompression of the syrinx and relieve compression on surrounding nerve fibers and blood vessels. Treatment aims to address either the filling mechanism of the syrinx or direct drainage of the syrinx fluid. The latter consists of syringostomy [20, 22, 23], cordectomy [21, 24], and syringosubarachnoid [16, 20, 23, 25], syringopleural [26, 27], and syringoperitoneal shunts [14, 28, 29]. In recent decades, CSF drainage techniques have been augmented by arachnoid lysis [21, 30], extradural decompression [31], and duraplasty [21, 30, 32]. However, no surgical technique has emerged superior [33] and long-term improvement occur in fewer than half of patients treated [17].
In the case of our patient, the original ballistic injury and retained bullet fragment deserve additional consideration. In the US, there are ~115,000 firearm injuries annually [34]. In most cases, bullet fragments are not routinely removed [35]. The proportion of SCIs caused by gunshot wounds are increasing [36]. Despite this burden, there are few described cases of PTS in the context of ballistic injury. In a systematic review of patients with gunshot injuries to the spinal cord, two of 26 required surgical intervention for PTS [37]. In the setting of ballistic injury in three cases, symptomatic syrinx formation has been reported at 6–8 years after the initial injury [22, 38], compared to 14-years in this report. All three of these cases required syrinx decompression. Beckley et al. described a similar operative approach with multi-level laminectomy, ballistic removal, cord untethering and syrinx drainage that yielded an improvement in neuropathic pain and recovery of sensory and motor loss at 3-months [39]. Similarly, our patient demonstrated improvement in neuropathic pain and motor function. MRI 2-weeks after surgery revealed reduction of syrinx diameter at the level of direct decompression. Syrinxes may take months to years to diminish in size after surgical decompression [40]; therefore, we are optimistic that the residual syrinx rostral to the level of decompression will continue to shrink in diameter and length.
Albeit without bullet removal, the surgical approach used in our case resembled techniques previously described by others [26, 31]. In 16 patients with complete cord trauma (ASIA A or B) that mostly underwent arachnolysis, untethering and duraplasty, Klekamp et al. demonstrated that 66% of patients improved and 35% had unchanged symptomology at 3-months, while 14% had clinical recurrence [21]. Alternatively, Ushewokunze et al. retrospectively reviewed 40 patients with PTS that had undergone multi-level laminectomy and arachnolysis [32]. Despite limited documented follow-up, this study found postoperative neurological deterioration in 41.4%, 32% and 37.5% at 6-weeks, 6-months and 1-year, respectively. Half of the patients in this study underwent further operations, including 18 shunt insertions (2 in 3), leading to the authors to conclude that decompression and arachnolysis have limited effect on long-term symptoms.
PTS remains a rare sequala of SCI. Despite being described in the literature, there remains a lack of consensus in management strategies. As described here, retained ballistic fragments adjacent to the spinal cord further complicate neurosurgical care. While this case report adds to existing literature on this rare disorder, results are based on retrospective findings, limiting generalization at the population level. Therefore, prospective, multi-center studies with large patient cohorts are required to improve prognostication and treatment options.
Conclusions
Definitive operative management of patients with PTS remains undetermined. Ballistic injury with retained fragments may further complicate approaches to decompress the syrinx. Our study adds to existing literature that extradural decompression and cord untethering helps facilitate restoration of physiological CSF flow.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Lasfargues JE, Custis D, Morrone F, Carswell J, Nguyen T. A model for estimating spinal cord injury prevalence in the United States. Paraplegia. 1995;33:62–8.
Schlesinger H. Die Syringomyelie: Eine Monographie. Leipzig und Wien: Franz Deuticke; 1895.
Hida K, Iwasaki Y, Imamura H, Abe H. Posttraumatic syringomyelia: its characteristic magnetic resonance imaging findings and surgical management. Neurosurgery. 1994;35:886–91.
Elliott NS, Lockerby DA, Brodbelt AR. A lumped-parameter model of the cerebrospinal system for investigating arterial-driven flow in posttraumatic syringomyelia. Med Eng Phys. 2011;33:874–82.
Mitchell JK (editor). Incomplete sections of nerves; injuries of the spinal cord and its neighborhood. In: Remote consequences of injuries of nerves and their treatment. Philadelphia: Lea Brothers & Co; 1895. p. 114–7.
Wagner W, Stolper P. Die Verletzungen der WirbelsaÈule und des RuÈckenmarks. Stuttgart: Verlag von Ferdinand Enke; 1898. p. 111–25.
National Spinal Cord Injury Statistical Center. 2020 Annual statistical report for the spinal cord injury model systems—complete public version. Birmingham, Alabama: University of Alabama at Birmingham; 2020.
Bonfield CM, Levi AD, Arnold PM, Okonkwo DO. Surgical management of post-traumatic syringomyelia. Spine. 2010;35:S245–58.
Krebs J, Koch HG, Hartmann K, Frotzler A. The characteristics of posttraumatic syringomyelia. Spinal Cord. 2016;54:463–6.
Kim HG, Oh HS, Kim TW, Park KH. Clinical features of post-traumatic syringomyelia. Korean J Neurotrauma. 2014;10:66–9.
Anton HA, Schweigel JF. Posttraumatic syringomyelia: the British Columbia experience. Spine. 1986;11:865–8.
Asano M, Fujiwara K, Yonenobu K, Hiroshima K. Post-traumatic syringomyelia. Spine. 1996;21:1446–53.
Goldstein B, Hammond MC, Stiens SA, Little JW. Posttraumatic syringomyelia: profound neuronal loss, yet preserved function. Arch Phys Med Rehabil. 1998;79:107–12.
Carroll AM, Brackenridge P. Post-traumatic syringomyelia: a review of the cases presenting in a regional spinal injuries unit in the north east of England over a 5-year period. Spine. 2005;30:1206–10.
Lee TT, Arias JM, Andrus HL, Quencer RM, Falcone SF, Green BA, et al. Progressive posttraumatic myelomalacic myelopathy: treatment with untethering and expansive duraplasty. J Neurosurg. 1997;86:624–8.
Rossier AB, Foo D, Shillito J, Dyro FM. Posttraumatic cervical syringomyelia. Incidence, clinical presentation, electrophysiological studies, syrinx protein and results of conservative and operative treatment. Brain. 1985;108:439–61.
Brodbelt AR, Stoodley MA. Post-traumatic syringomyelia: a review. J Clin Neurosci. 2003;10:401–8.
Timpone VM, Patel SH. MRI of a syrinx: is contrast material always necessary? Am J Roentgenol. 2015;204:1082–5.
Ronen J, Catz A, Spasser R, Gepstein R. The treatment dilemma in post-traumatic syringomyelia. Disabil Rehabil. 1999;21:455–7.
Schurch B, Wichmann W, Rossier AB. Post-traumatic syringomyelia (cystic myelopathy): a prospective study of 449 patients with spinal cord injury. J Neurol Neurosurg Psychiatry. 1996;60:61–7.
Klekamp J. Treatment of posttraumatic syringomyelia. J Neurosurg Spine. 2012;17:199–211.
Shannon N, Symon L, Logue V, Cull D, Kang J, Kendall B, et al. Clinical features, investigation and treatment of post-traumatic syringomyelia. J Neurol Neurosurg Psychiatry. 1981;44:35–42.
Tator CH, Meguro K, Rowed DW. Favorable results with syringosubarachnoid shunts for treatment of syringomyelia. J Neurosurg. 1982;56:517–23.
Laxton AW, Perrin RG. Cordectomy for the treatment of posttraumatic syringomyelia. Report of four cases and review of the literature. J Neurosurg Spine. 2006;4:174–8.
Edgar R, Quail P. Progressive post-traumatic cystic and non-cystic myelopathy. Br J Neurosurg. 1994;8:7–22.
Sgouros S, Williams B. A critical appraisal of drainage in syringomyelia. J Neurosurg. 1995;82:1–10.
Cacciola F, Capozza M, Perrini P, Benedetto N, Di Lorenzo N. Syringopleural shunt as a rescue procedure in patients with syringomyelia refractory to restoration of cerebrospinal fluid flow. Neurosurgery. 2009;65:471–6.
Suzuki M, Davis C, Symon L, Gentili F. Syringoperitoneal shunt for treatment of cord cavitation. J Neurol Neurosurg Psychiatry. 1985;48:620–7.
Lam S, Batzdorf U, Bergsneider M. Thecal shunt placement for treatment of obstructive primary syringomyelia. J Neurosurg Spine. 2008;9:581–8.
Falci SP, Indeck C, Lammertse DP. Posttraumatic spinal cord tethering and syringomyelia: surgical treatment and long-term outcome. J Neurosurg Spine. 2009;11:445–60.
Holly LT, Johnson JP, Masciopinto JE, Batzdorf U. Treatment of posttraumatic syringomyelia with extradural decompressive surgery. Neurosurg Focus. 2000;8:E8.
Ushewokunze SO, Gan YC, Phillips K, Thacker K, Flint G. Surgical treatment of post-traumatic syringomyelia. Spinal Cord. 2010;48:710–3.
Kleindienst A, Engelhorn T, Roeckelein V, Buchfelder M. Development of pre-syrinx state and syringomyelia following a minor injury: a case report. J Med Case Rep. 2020;14:223.
Gotsch KE, Annest JL, Mercy JA, Ryan GW. Surveillance for fatal and nonfatal firearm-related injuries—United States, 1993–1998. MMWR. 2001;50:1–32.
McQuirter JL, Rothenberg SJ, Dinkins GA, Manalo M, Kondrashov V, Todd AC. The effects of retained lead bullets on body lead burden. J Trauma. 2001;50:892–9.
Waters RL, Sie IH. Spinal cord injuries from gunshot wounds to the spine. Clin Orthop Relat Res. 2003;408:120–5.
Sidhu GS, Ghag A, Prokuski V, Vaccaro AR, Radcliff KE. Civilian gunshot injuries of the spinal cord: a systematic review of the current literature. Clin Orthop Relat Res. 2013;471:3945–55.
Vernon JD, Silver JR, Ohry A. Post-traumatic syringomyelia. Paraplegia. 1982;20:339–64.
Beckley AO, Oleson CV, Formal CS. Development of syringomyelia from retained bullet fragment following spinal cord injury. Toronto, Canada: American Congress of Rehabilitation Medicine; Department of Rehabilitation Medicine Faculty Papers; 2014.
Wetjen NM, Heiss JD, Oldfield EH. Time course of syringomyelia resolution following decompression of Chiari malformation Type I. J Neurosurg Pediatr. 2008;1:118–23.
Acknowledgements
The authors thank Eleanor Buckle who provided valuable assistance with technical aspects of this project.
Author information
Authors and Affiliations
Contributions
TDA and NT conceived of the presented idea. TDA and JM wrote the manuscript with support from BYH, DM, KRL, LH, LJB, YX, PPS, NVK, and NT. NT supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Ethical approval was not required by the Johns Hopkins Medicine Institutional Review Board, as the presented work is a medical/educational activity that does not meet the Department of Health and Human Services definition of “research”, which is: “a systematic investigation, including research development, testing and evaluation, designed to develop or contribute to generalizable knowledge.”
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Azad, T.D., Materi, J., Hwang, B.Y. et al. Spinal cord untethering and midline myelotomy for delayed, symptomatic post-traumatic syringomyelia due to retained ballistic fragments: case report. Spinal Cord Ser Cases 8, 66 (2022). https://doi.org/10.1038/s41394-022-00533-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41394-022-00533-7