Abstract
Introduction
In patients with metastatic hormone-sensitive prostate cancer (mHSPC) undergoing intensified androgen deprivation therapy (ADT), not achieving an optimal PSA response, defined as PSA nadir >0.2 ng/ml (PSAsubOR) has been associated with worse survival outcomes in clinical trials (1)(10)(11). Here, we externally evaluate, the impact of optimal PSA response on survival outcomes in these patients and provide absolute PFS and OS measures in those with PSAsubOR in the context of ADT intensification in real world setting.
Methods
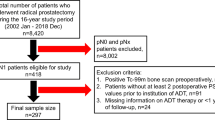
In this retrospective study, all consecutive patients with mHSPC who underwent intensified ADT treated at our institution, and whose outcomes data were available, were included. We classified patients based on their PSA nadir on treatment: those with a on treatment PSAOR (PSA nadir ≤0.2 ng/ml) versus PSAsubOR.
Results
A total of 205 patients were eligible: 136 (66.3%) patients achieved PSAOR versus 69 (33.7%) patients had PSAsubOR. Patients who experienced a PSAOR had significantly improved PFS and OS from the start of intensified ADT versus who did not: PFS was not reached (NR) versus 11 months (hazard ratio (HR) 0.20, P < 0.001) and OS was NR versus 38.9 months (HR 0.21, P < 0.001). Survival outcomes were poor with PSAsubOR regardless of intensification with docetaxel or an ARPI (absolute PFS and OS measures for each group are provided in the text).
Conclusion
Our study is the first to explore the negative impact of PSAsubOR in patients with mHSPC undergoing intensified ADT in the real-world setting, and is the first to provide absolute PFS and OS in patients with PSAsubOR receiving ADT intensification with ARPIs or docetaxel outside of clinical trial setting. These data will aid with prognostication, patient counseling, and for designing future clinical trials for patients with PSAsubOR.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The data generated in this study are not publicly available as they could compromise patient privacy but are available upon reasonable request from the corresponding author.
Change history
24 August 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41391-023-00706-x
References
Smith MR, Hussain M, Saad F, Fizazi K, Sternberg CN, Crawford ED, et al. Darolutamide and survival in metastatic, hormone-sensitive prostate cancer. N. Engl J Med. 2022;386:1132–42.
Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972;22:232–40.
Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, Eisenberger M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N. Engl J Med. 2015;373:737–46.
James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163–77.
Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20:686–700.
Chi KN, Agarwal N, Bjartell A, Chung BH, Pereira de Santana Gomes AJ, Given R, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N. Engl J Med. 2019;381:13–24.
Armstrong AJ, Szmulewitz RZ, Petrylak DP, Holzbeierlein J, Villers A, Azad A, et al. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. JCO. 2019;37:2974–86.
Hussain M, Tangen CM, Higano C, Schelhammer PF, Faulkner J, Crawford ED, et al. Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: data from Southwest Oncology Group Trial 9346 (INT-0162). J Clin Oncol. 2006;24:3984–90.
Chowdhury S, Bjartell A, Agarwal N, Chung BH, Given RW, Gomes AJP et al. Deep, rapid, and durable prostate-specific antigen decline with apalutamide plus androgen deprivation therapy is associated with longer survival and improved clinical outcomes in TITAN patients with metastatic castration-sensitive prostate cancer. Annals of Oncology [Internet]. 2023 Feb [cited 2023 Mar 6];0. Available from: https://www.annalsofoncology.org/article/S0923-7534 (23)00086-8/fulltext
Saad F, Hussain MHA, Tombal BF, Fizazi K, Sternberg CN, Crawford ED, et al. Association of prostate-specific antigen (PSA) response and overall survival (OS) in patients with metastatic hormone-sensitive prostate cancer (mHSPC) from the phase 3 ARASENS trial. JCO. 2022;40:5078–5078.
Matsubara N, Chi KN, Özgüroğlu M, Rodriguez-Antolin A, Feyerabend S, Fein L, et al. Correlation of prostate-specific antigen kinetics with overall survival and radiological progression-free survival in metastatic castration-sensitive prostate cancer treated with abiraterone acetate plus prednisone or placebos added to androgen deprivation therapy: post hoc analysis of phase 3 latitude study. Eur Urol. 2020;77:494–500.
Halabi S, Roy A, Guo SS, Rydzewska L, Godolphin P, Hussain MHA, et al. Assessing PSA levels as prognostic of overall survival (OS) in men with metastatic hormone-sensitive prostate cancer (mHSPC). JCO. 2023;41:5070–5070.
Harshman LC, Chen YH, Liu G, Carducci MA, Jarrard D, Dreicer R, et al. Seven-month prostate-specific antigen is prognostic in metastatic hormone-sensitive prostate cancer treated with androgen deprivation with or without docetaxel. J Clin Oncol. 2018;36:376–82.
Sheldrick RC. Randomized trials vs real-world evidence: how can both inform decision-making? JAMA. 2023;329:1352–3.
Author information
Authors and Affiliations
Contributions
Study concept and design: GG, NS, VMT, BLM, US, NA. Acquisition of data: GG, NS, VMT, NT, HL, KKS, AS2, TM, BLM, US, NA. Analysis and interpretation of data: GG, NS, VMT, BC, YJ, US, NA. Drafting of the manuscript: GG, NS, VMT. Critical revision of the manuscript for important intellectual content: GG, NS, VMT, NT, BLM, US, NA. Supervision: US, NA.
Corresponding author
Ethics declarations
Competing interests
Neeraj Agarwal, MD: NA does not report any personal COI since April 15, 2021. However, the following are his lifetime personal COIs: Consultancy to Astellas, Astra Zeneca, Aveo, Bayer, Bristol Myers Squibb, Calithera, Clovis, Eisai, Eli Lilly, EMD Serono, Exelixis, Foundation Medicine, Genentech, Gilead, Janssen, Merck, MEI Pharma, Nektar, Novartis, Pfizer, Pharmacyclics, and Seattle Genetics. Research funding to Neeraj Agarwal’s institution: Arnivas, Astellas, Astra Zeneca, Bavarian Nordic, Bayer, Bristol Myers Squibb, Calithera, Celldex, Clovis, Crispr, Eisai, Eli Lilly, EMD Serono, Exelixis, Genentech, Gilead, Glaxo Smith Kline, Immunomedics, Janssen, Lava, Medivation, Merck, Nektar, Neoleukin, New Link Genetics, Novartis, Oric, Pfizer, Prometheus, Rexahn, Roche, Sanofi, Seattle Genetics, Takeda, and Tracon. Umang Swami, MD: has been paid for a consulting or advisory role by Seattle Genetics, Astellas Pharma, Exelixis, Imvax, and AstraZeneca, currently or during the past 2 years. Dr. Swami’s institution has received research funding from Janssen, Seattle Genetics/Astellas, and Exelixis, currently or within the past 2 years. Benjamin L. Maughan, MD: is a paid consultant/advisor to Abbvie, Pfizer, AVEO oncology, Janssen, Astellas, Bristol-Myers Squibb, Clovis, Tempus, Merck, Exelixis, Bayer Oncology and Peloton Therapeutics; Huntsman Cancer Institute has received research funding from Exelixis (Inst), Bavarian-Nordic (Inst), Clovis (Inst), Genentech (Inst) and Bristol-Myers Squibb (Inst) on his behalf.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gebrael, G., Sayegh, N., Thomas, V.M. et al. Survival outcomes of real world patients with metastatic hormone-sensitive prostate cancer who do not achieve optimal PSA response with intensified androgen deprivation therapy with docetaxel or androgen receptor pathway inhibitors. Prostate Cancer Prostatic Dis (2023). https://doi.org/10.1038/s41391-023-00696-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41391-023-00696-w