Abstract
Background
The site of prostate cancer metastasis is an important predictor of oncologic outcomes, however, the clinicogenomic characteristics associated with the site are not well-defined. Herein, we characterize the genomic alterations associated with the metastatic site of prostate cancer.
Methods
We analyzed clinical and genomic data from prostate cancer patients with metastatic disease and known metastatic sites from publicly available targeted sequencing data.
Results
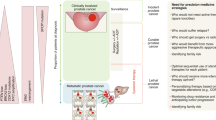
Prostate cancer metastasis to the liver versus other sites of metastasis conferred a high hazard for death in patients with metastatic prostate cancer (HR: 3.96, 95% CI: 2.4–6.5, p < 0.0001). Genomic analysis of metastatic tissues of prostate cancer-specific genes demonstrated that liver metastases were more enriched with MYC amplification (29.5% vs. 9.8%, FDR = 0.001), PTEN deletion (42% vs. 20.8%, FDR = 0.005), and PIK3CB amplification (8.2% vs. 0.9, FDR = 0.005) compared to other sites. No point mutations were significantly associated with liver metastasis compared to other metastatic sites.
Conclusion
Liver metastases in prostate cancer are associated with poor survival and aggressive genomic features, including MYC-amplification, PTEN-deletion, and PIK3CB-amplification. These findings could have prognostic, treatment, and trial implications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Halabi S, Kelly WK, Ma H, Zhou H, Solomon NC, Fizazi K, et al. Meta-analysis evaluating the impact of site of metastasis on overall survival in men with castration-resistant prostate cancer. J Clin Oncol. 2016;34:1652–9.
Pond GR, Sonpavde G, De Wit R, Eisenberger MA, Tannock IF, Armstrong AJ. The prognostic importance of metastatic site in men with metastatic castration-resistant prostate cancer. Eur Urol. 2014;65:3–6.
Zhao F, Wang J, Chen M, Chen D, Ye S, Li X, et al. Sites of synchronous distant metastases and prognosis in prostate cancer patients with bone metastases at initial diagnosis: a population-based study of 16,643 patients. Clin Transl Med. 2019;8:30.
Singh A, Cheedella NKS, Shakil SA, Gulmi F, Kim DS, Wang JC. Liver metastases in prostate carcinoma represent a relatively aggressive subtype refractory to hormonal therapy and short-duration response to docetaxel monotherapy. Can J Addict Med. 2015;6:265–9.
Pouessel D, Gallet B, Bibeau F, Avancès C, Iborra F, Sénesse P, et al. Liver metastases in prostate carcinoma: Clinical characteristics and outcome. BJU Int. 2007;99:807–11.
Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23:703–13.
Nguyen B, Mota JM, Nandakumar S, Stopsack KH, Weg E, Rathkopf D, et al. Pan-cancer Analysis of CDK12 alterations identifies a subset of prostate cancers with distinct genomic and clinical characteristics. Eur Urol. 2020. https://doi.org/10.1016/j.eururo.2020.03.024.
Annala M, Vandekerkhove G, Khalaf D, Taavitsainen S, Beja K, Warner EW, et al. Circulating tumor DNA genomics correlate with resistance to abiraterone and enzalutamide in prostate cancer. Cancer Discov. 2018;8:444–57.
Koh CM, Iwata T, Zheng Q, Bethel C, Yegnasubramanian S, De Marzo AM. Myc enforces overexpression of EZH2 in early prostatic neoplasia via transcriptional and post-transcriptional mechanisms. Oncotarget. 2011;2:669–83.
Labbé DP, Sweeney CJ, Brown M, Galbo P, Rosario S, Wadosky KM, et al. TOP2A and EZH2 provide early detection of an aggressive prostate cancer subgroup. Clin Cancer Res. 2017;23:7072–83.
Kim KH, Roberts CWM. Targeting EZH2 in cancer. Nat Med. 2016;22:128–34.
LoRusso PM. Inhibition of the PI3K/AKT/mTOR pathway in solid tumors. J Clin Oncol. 2016;34:3803–15.
Funding
Dr. Mahal is funded by the Prostate Cancer Foundation, American Society for Radiation Oncology, Department of Defense, and the Sylvester Comprehensive Cancer Center.
Author information
Authors and Affiliations
Contributions
MA, CS, IF, and BM conducted the analysis, drafted the paper, and contributed to revisions. MA and AP contributed to data acquisition and critical revision for important content. RV, RC, SP, SK, RD, AK, DS, JS, and ADP contributed to the conception of the study, data acquisition, and critical revision for important content. MA, CS, and BM have responsibility for the final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
Dr. Mahal is funded by the Prostate Cancer Foundation-American Society for Radiation Oncology Award to End Prostate Cancer (grant support). Dr. Franco is funded by an NIH NCI Center to Reduce Cancer Health Disparities Diversity Supplement, and ACRO Luther Brady Educational Award (grant support). Dr. Spratt has received personal fees: Janssen, Blue Earth, Boston Scientific, AstraZeneca, and funding from Janssen. The remaining authors declare that they have no conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Alshalalfa, M., Seldon, C., Franco, I. et al. Clinicogenomic characterization of prostate cancer liver metastases. Prostate Cancer Prostatic Dis 25, 366–369 (2022). https://doi.org/10.1038/s41391-021-00486-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-021-00486-2
This article is cited by
-
From biology to the clinic — exploring liver metastasis in prostate cancer
Nature Reviews Urology (2024)
-
Autophagy, a critical element in the aging male reproductive disorders and prostate cancer: a therapeutic point of view
Reproductive Biology and Endocrinology (2023)
-
Incidence and survival of castration-resistant prostate cancer patients with visceral metastases: results from the Dutch CAPRI-registry
Prostate Cancer and Prostatic Diseases (2023)