Abstract
Background
Several definitions have attempted to stratify metastatic castrate-sensitive prostate cancer (mCSPC) into low and high-volume states. However, at this time, comparison of these definitions is limited. Here we aim to compare definitions of metastatic volume in mCSPC with respect to clinical outcomes and mutational profiles.
Methods
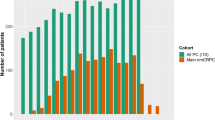
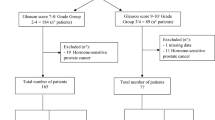
We performed a retrospective review of patients with biochemically recurrent or mCSPC whose tumors underwent somatic targeted sequencing. 294 patients were included with median follow-up of 58.3 months. Patients were classified into low and high-volume disease per CHAARTED, STAMPEDE, and two numeric (≤3 and ≤5) definitions. Endpoints including radiographic progression-free survival (rPFS), time to development of castration resistance (tdCRPC), and overall survival (OS) were evaluated with Kaplan–Meier survival curves and log-rank test. The incidence of driver mutations between definitions were compared.
Results
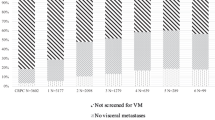
Median OS and tdCRPC were shorter for high-volume than low-volume disease for all four definitions. In the majority of patients (84.7%) metastatic volume classification did not change across all four definitions. High volume disease was significantly associated with worse OS for all four definitions (CHAARTED: HR 2.89; p < 0.01, STAMPEDE: HR 3.82; p < 0.01, numeric ≤3: HR 4.67; p < 0.01, numeric ≤5: HR 3.76; p < 0.01) however, were similar for high (p = 0.95) and low volume (p = 0.79) disease across all four definitions. Those with discordant classification tended to have more aggressive clinical behavior and mutational profiles. Patients with low-volume disease and TP53 mutation experienced a more aggressive course with rPFS more closely mirroring high-volume disease.
Conclusions
The spectrum of mCSPC was confirmed across four different metastatic definitions for clinical endpoints and genetics. All definitions were generally similar in classification of patients, outcomes, and genetic makeup. Given these findings, the simplicity of numerical definitions might be preferred, especially when integrating metastasis directed therapy. Incorporation of tumor genetics may allow further refinement of current metastatic definitions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13:8–10.
Kyriakopoulos CE, Chen YH, Carducci MA, Liu G, Jarrard DF, Hahn NM, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED Trial. J Clin Oncol. 2018;36:1080–7.
James ND, de Bono JS, Spears MR, Clarke NW, Mason MD, Dearnaley DP, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377:338–51.
Parker CC, James ND, Brawley CD, Clarke NW, Hoyle AP, Ali A, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet 2018;392:2353–66.
Phillips R, Shi WY, Deek M, Radwan N, Lim SJ, Antonarakis ES, et al. Outcomes of observation vs stereotactic ablative radiation for oligometastatic prostate cancer: The ORIOLE phase 2 randomized clinical trial. JAMA Oncol. 2020;6:650–9.
Gomez DR, Tang C, Zhang J, Blumenschein GR Jr, Hernandez M, Lee JJ, et al. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol. 2019;37:1558–65.
Sutera P, Clump DA, Kalash R, D’Ambrosio D, Mihai A, Wang H, et al. Initial results of a multicenter phase 2 trial of stereotactic ablative radiation therapy for oligometastatic cancer. Int J Radiat Oncol Biol Phys. 2019;103:116–22.
Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet 2019;393:2051–8.
Ost P, Reynders D, Decaestecker K, Fonteyne V, Lumen N, De Bruycker A, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36:446–53.
Ali A, Hoyle A, Haran ÁM, Brawley CD, Cook A, Amos C, et al. Association of bone metastatic burden with survival benefit from prostate radiotherapy in patients with newly diagnosed metastatic prostate cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2021;7:555–63.
Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the prostate cancer clinical trials working group 3. J Clin Oncol. 2016;34:1402–18.
Pasoglou V, Michoux N, Van Damme J, Van Nieuwenhove S, Halut M, Triqueneaux P, et al. Pattern of metastatic deposit in recurrent prostate cancer: a whole-body MRI-based assessment of lesion distribution and effect of primary treatment. World J Urol. 2019;37:2585–95.
Gupta SK, Watson T, Denham J, Shakespeare TP, Rutherford N, McLeod N, et al. Prostate-specific membrane antigen positron emission tomography-computed tomography for prostate cancer: distribution of disease and implications for radiation therapy planning. Int J Radiat Oncol Biol Phys. 2017;99:701–9.
Ost P, Reynders D, Decaestecker K, Fonteyne V, Lumen N, Bruycker AD, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence (STOMP): Five-year results of a randomized phase II trial. J Clin Oncol. 2020;38:10.
Abida W, Armenia J, Gopalan A, Brennan R, Walsh M, Barron D, et al. Prospective genomic profiling of prostate cancer across disease states reveals germline and somatic alterations that may affect clinical decision making. JCO Precis Oncol. 2017;2017.
Abida W, Cyrta J, Heller G, Prandi D, Armenia J, Coleman I, et al. Genomic correlates of clinical outcome in advanced prostate cancer. Proc Natl Acad Sci USA. 2019;116:11428–36.
Hamid AA, Gray KP, Shaw G, MacConaill LE, Evan C, Bernard B, et al. Compound genomic alterations of TP53, PTEN, and RB1 tumor suppressors in localized and metastatic prostate cancer. Eur Urol. 2019;76:89–97.
Baca SC, Prandi D, Lawrence MS, Mosquera JM, Romanel A, Drier Y, et al. Punctuated evolution of prostate cancer genomes. Cell 2013;153:666–77.
Boutros PC, Fraser M, Harding NJ, de Borja R, Trudel D, Lalonde E, et al. Spatial genomic heterogeneity within localized, multifocal prostate cancer. Nat Genet. 2015;47:736–45.
Fraser M, Sabelnykova VY, Yamaguchi TN, Heisler LE, Livingstone J, Huang V, et al. Genomic hallmarks of localized, non-indolent prostate cancer. Nature 2017;541:359–64.
Castro E, Romero-Laorden N, Del Pozo A, Lozano R, Medina A, Puente J, et al. PROREPAIR-B: a prospective cohort study of the impact of germline DNA repair mutations on the outcomes of patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2019;37:490–503.
Beltran H, Prandi D, Mosquera JM, Benelli M, Puca L, Cyrta J, et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat Med. 2016;22:298–305.
Aparicio AM, Shen L, Tapia EL, Lu JF, Chen HC, Zhang J, et al. Combined tumor suppressor defects characterize clinically defined aggressive variant prostate cancers. Clin Cancer Res. 2016;22:1520–30.
De Laere B, Oeyen S, Mayrhofer M, Whitington T, van Dam PJ, Van Oyen P, et al. TP53 outperforms other androgen receptor biomarkers to predict abiraterone or enzalutamide outcome in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2019;25:1766–73.
Deek MP, Van der Eecken K, Phillips R, Parikh NR, Isaacsson Velho P, Lotan TL, et al. The mutational landscape of metastatic castration-sensitive prostate cancer: The Spectrum Theory Revisited. Eur Urol. 2021;80:632–64.
Schweizer MT, Sivakumar S, Tukachinsky H, Coleman I, De Sarkar N, Yu EY, et al. Concordance of DNA repair gene mutations in paired primary prostate cancer samples and metastatic tissue or cell-free DNA. JAMA Oncol. 2021;7:1–5.
Author information
Authors and Affiliations
Contributions
Study concept and design: PS, PTT, MPD. Acquisition of data: PS, PTT, MPD. Drafting of manuscript: PS, PTT, MPD. Critical review of manuscript: PS, KVDE, AUK, AH, EG, GA, TL, AAM, CJP, MAC, AR, HW, KP, FYF, ESA, PO, DYS, SG, CD, TD, PTT, MPD.
Corresponding author
Ethics declarations
Competing interests
Consultant for Pfizer, Exelexis. ESA: Patent holder/licenser for Qiagen; Consultant for Amgen, Astellas, AstraZeneca, Bayer, Clovis, Dendreon, Eli Lilly and Co. ESSA, GlaxoSmithKline, Jannsen, Medivation, Merck, and Sanofi; Research grant recipient from AstraZeneca, Bristol Myers-Squibb, Celgene, Clovis, Dendreon, Genentech, Janssen, Johnson & Johnson, Merck, Novartis, Sanofi, Tokai. PO: Consultant for Bayer, Janssen, Curium; Research grant recipient from Varian, Bayer. AG: Relationship with Janssen and Astellas; Royalties from ICR. AR: Speaker’s Bureau for Blue Earth and Janssen; Stock Options from Decipher Biosciences; Consultant to Astellas. PT: Research funding from Astellas Pharm, Bayer Healthcare and RefleXion Medial Inc; Consultant for RefleXion, Grants from RefleXion; Personal fees from Noxopharm, Janssen-Taris Biomedical, Myovant and AstraZeneca; Holds a patent 9114158- Compounds and Methods of Use in Ablative Radiotherapy licensed to Natsar Pharm.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Sutera, P., Van Der Eecken, K., Kishan, A.U. et al. Definitions of disease burden across the spectrum of metastatic castration-sensitive prostate cancer: comparison by disease outcomes and genomics. Prostate Cancer Prostatic Dis 25, 713–719 (2022). https://doi.org/10.1038/s41391-021-00484-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-021-00484-4
This article is cited by
-
Diagnostik und Stadieneinteilung beim metastasierten hormonsensitiven Prostatakarzinom
Urologie in der Praxis (2023)
-
Diagnostik und Stadieneinteilung beim metastasierten hormonsensitiven Prostatakarzinom
Die Urologie (2023)
-
Prostate and metastasis diffusion volume based on apparent diffusion coefficient as a prognostic factor in Hormone-naïve prostate Cancer
Clinical & Experimental Metastasis (2023)
-
The oligometastatic spectrum in the era of improved detection and modern systemic therapy
Nature Reviews Clinical Oncology (2022)