Abstract
Background
To determine whether combining brachytherapy with immunotherapy is safe in prostate cancer (PCa) and provides synergistic effects, we performed a Phase I/II trial on the feasibility, safety, and benefit of concurrent delivery of anti-PD-1 (nivolumab) with high-dose-rate (HDR) brachytherapy and androgen deprivation therapy (ADT) in patients with Grade Group 5 (GG5) PCa.
Methods
Eligible patients were aged 18 years or older with diagnosis of GG5 PCa. Patients received ADT, nivolumab every two weeks for four cycles, with two cycles prior to first HDR, and two more cycles prior to second HDR, followed by external beam radiotherapy. The primary endpoint was to determine safety and feasibility. This Phase I/II trial is registered with ClinicalTrials.gov (NCT03543189).
Results
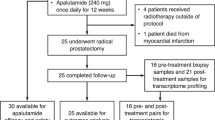
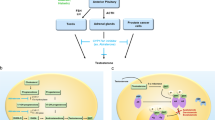
Between September 2018 and June 2019, six patients were enrolled for the Phase I safety lead-in with a minimum observation period of 3 months after nivolumab administration. Overall, nivolumab was well tolerated in combination with ADT and HDR treatment. One patient experienced a grade 3 dose-limiting toxicity (elevated Alanine aminotransferase and Aspartate aminotransferase) after the second cycle of nivolumab. Three patients (50%) demonstrated early response with no residual tumor detected in ≥4 of 6 cores on biopsy post-nivolumab (4 cycles) and 1-month post–HDR. Increase in CD8+ and FOXP3+/CD4+ T cells in tissues, and CD4+ effector T cells in peripheral blood were observed in early responders.
Conclusion
Combination of nivolumab with ADT and HDR is well tolerated and associated with evidence of increased immune infiltration and antitumor activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl J Med. 2017;377:1345–56.
Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl J Med. 2015;372:2018–28.
Motzer RJ, Tannir NM, McDermott DF, Aren Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N. Engl J Med. 2018;378:1277–90.
Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N. Engl J Med. 2017;376:1015–26.
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl J Med. 2012;366:2443–54.
Graff JN, Alumkal JJ, Drake CG, Thomas GV, Redmond WL, Farhad M, et al. Early evidence of anti-PD-1 activity in enzalutamide-resistant prostate cancer. Oncotarget. 2016;7:52810–7.
D’Amico AV, Chen MH, Renshaw AA, Loffredo M, Kantoff PW. Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. JAMA. 2008;299:289–95.
Denham JW, Steigler A, Lamb DS, Joseph D, Turner S, Matthews J, et al. Short-term neoadjuvant androgen deprivation and radiotherapy for locally advanced prostate cancer: 10-year data from the TROG 96.01 randomised trial. Lancet Oncol. 2011;12:451–9.
D’Amico AV. Is Gleason grade 5 prostate cancer resistant to conventional androgen deprivation therapy? Eur Urol. 2016;69:761–3.
Chakraborty M, Abrams SI, Coleman CN, Camphausen K, Schlom J, Hodge JW. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 2004;64:4328–37.
Gameiro SR, Malamas AS, Bernstein MB, Tsang KY, Vassantachart A, Sahoo N, et al. Tumor cells surviving exposure to proton or photon radiation share a common immunogenic modulation signature, rendering them more sensitive to T cell-mediated killing. Int J Radiat Oncol Biol Phys. 2016;95:120–30.
Gameiro SR, Ardiani A, Kwilas A, Hodge JW. Radiation-induced survival responses promote immunogenic modulation to enhance immunotherapy in combinatorial regimens. Oncoimmunology. 2014;3:e28643.
Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–7.
Patel RB, Baniel CC, Sriramaneni RN, Bradley K, Markovina S, Morris ZS. Combining brachytherapy and immunotherapy to achieve in situ tumor vaccination: A review of cooperative mechanisms and clinical opportunities. Brachytherapy. 2018;17:995–1003.
Walle T, Martinez Monge R, Cerwenka A, Ajona D, Melero I, Lecanda F. Radiation effects on antitumor immune responses: current perspectives and challenges. Therapeutic Adv Med Oncol. 2018;10:1758834017742575.
Koh YT, Gray A, Higgins SA, Hubby B, Kast WM. Androgen ablation augments prostate cancer vaccine immunogenicity only when applied after immunization. Prostate. 2009;69:571–84.
Roden AC, Moser MT, Tri SD, Mercader M, Kuntz SM, Dong H, et al. Augmentation of T cell levels and responses induced by androgen deprivation. J Immunol. 2004;173:6098–108.
Aragon-Ching JB, Williams KM, Gulley JL. Impact of androgen-deprivation therapy on the immune system: implications for combination therapy of prostate cancer. Front Biosci: a J virtual Libr. 2007;12:4957–71.
Sanda MG, Smith DC, Charles LG, Hwang C, Pienta KJ, Schlom J, et al. Recombinant vaccinia-PSA (PROSTVAC) can induce a prostate-specific immune response in androgen-modulated human prostate cancer. Urology. 1999;53:260–6.
George DJ, Nabhan C, DeVries T, Whitmore JB, Gomella LG. Survival outcomes of sipuleucel-T phase III studies: impact of control-arm cross-over to salvage immunotherapy. Cancer Immunol Res. 2015;3:1063–9.
Small EJ, Lance RS, Gardner TA, Karsh LI, Fong L, McCoy C, et al. A randomized phase II trial of sipuleucel-T with concurrent versus sequential abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2015;21:3862–9.
Mercader M, Bodner BK, Moser MT, Kwon PS, Park ES, Manecke RG, et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci USA. 2001;98:14565–70.
Epstein JI, Zelefsky MJ, Sjoberg DD, Nelson JB, Egevad L, Magi-Galluzzi C, et al. A contemporary prostate cancer grading system: a validated alternative to the Gleason score. Eur Urol. 2016;69:428–35.
Nabid A, Carrier N, Martin AG, Bahary JP, Lemaire C, Vass S, et al. Duration of androgen deprivation therapy in high-risk prostate cancer: a randomized phase III trial. Eur Urol. 2018;74:432–41.
Matta R, Chapple CR, Fisch M, Heidenreich A, Herschorn S, Kodama RT, et al. Pelvic complications after prostate cancer radiation therapy and their management: an international collaborative narrative review. Eur Urol. 2019;75:464–76.
Som A, Mandaliya R, Alsaadi D, Farshidpour M, Charabaty A, Malhotra N, et al. Immune checkpoint inhibitor-induced colitis: a comprehensive review. World J Clin Cases. 2019;7:405–18.
Wang Y, Abu-Sbeih H, Mao E, Ali N, Ali FS, Qiao W, et al. Immune-checkpoint inhibitor-induced diarrhea and colitis in patients with advanced malignancies: retrospective review at MD Anderson. J Immunother Cancer. 2018;6:37.
Mahoney KM, Atkins MB. Prognostic and predictive markers for the new immunotherapies. Oncology. 2014;28:39–48.
Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–8.
Bilusic M, Madan RA, Gulley JL. Immunotherapy of prostate cancer: facts and hopes. Clin Cancer Res. 2017;23:6764–70.
Beer TM, Kwon ED, Drake CG, Fizazi K, Logothetis C, Gravis G, et al. Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J Clin Oncol: Off J Am Soc Clin Oncol. 2017;35:40–7.
Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:700–12.
Antonarakis ES, Piulats JM, Gross-Goupil M, Goh J, Ojamaa K, Hoimes CJ, et al. Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: multicohort, open-label phase II KEYNOTE-199 Study. J Clin Oncol 2019;38:395–405.
Gorbet MJ, Ranjan A. Cancer immunotherapy with immunoadjuvants, nanoparticles, and checkpoint inhibitors: recent progress and challenges in treatment and tracking response to immunotherapy. Pharmacol Ther 2019;207:107456.
Geara FB, Bulbul M, Khauli RB, Andraos TY, Abboud M, Al Mousa A, et al. Nadir PSA is a strong predictor of treatment outcome in intermediate and high risk localized prostate cancer patients treated by definitive external beam radiotherapy and androgen deprivation. Radiat Oncol. 2017;12:149.
Verma V, Shrimali RK, Ahmad S, Dai W, Wang H, Lu S, et al. PD-1 blockade in subprimed CD8 cells induces dysfunctional PD-1(+)CD38(hi) cells and anti-PD-1 resistance. Nat Immunol. 2019;20:1231–43.
Acknowledgements
The authors acknowledge Moffitt Cancer Center CLIA Tissue Imaging Lab for Multiplex Immune Panel Procedure and Quantitative Image Analysis.
Role of the funding source:
Bristol Myers Squibb (BMS) had no role in the study design, data collection, analysis, and interpretation, or writing of the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
J.Z. reports honorarium from AstraZeneca, Merck, Sanofi and Bayer for speaker programs and advisory boards. R.J. serves as an advisor for Pfizer and a speaker for Dava Oncology. R.L. is on Clinical trial protocol committee for Cold Genesys and BMS, and serves as a scientific advisor/consultant for BMS and Ferring. S.K. reports research funds from BMS and Astra Zeneca. The remaining authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Clinical trial: This Phase I/II trial is registered with ClinicalTrials.gov (NCT03543189).
Author responsible for statistical analysis: Youngchul Kim, PhD; Youngchul.Kim@moffitt.org
Funded by: Bristol Myers Squibb
Rights and permissions
About this article
Cite this article
Yuan, Z., Fernandez, D., Dhillon, J. et al. Proof-of-principle Phase I results of combining nivolumab with brachytherapy and external beam radiation therapy for Grade Group 5 prostate cancer: safety, feasibility, and exploratory analysis. Prostate Cancer Prostatic Dis 24, 140–149 (2021). https://doi.org/10.1038/s41391-020-0254-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-020-0254-y
This article is cited by
-
Radiotherapy combined with nano-biomaterials for cancer radio-immunotherapy
Journal of Nanobiotechnology (2023)
-
Radiotherapy combined with immunotherapy: the dawn of cancer treatment
Signal Transduction and Targeted Therapy (2022)
-
Immune-related lncRNAs as predictors of survival in breast cancer: a prognostic signature
Journal of Translational Medicine (2020)