Abstract
Background
Patients with high-risk prostate cancer have an increased likelihood of experiencing a relapse following radical prostatectomy (RP). We previously conducted three neoadjuvant androgen-deprivation therapy (ADT) trials prior to RP in unfavorable intermediate and high-risk disease.
Methods
In this analysis, we report on the post-RP outcomes of a subset of patients enrolled on these studies. We conducted a pooled analysis of patients with available follow-up data treated on three neoadjuvant trials at three institutions. All patients received intense ADT prior to RP. The primary endpoint was time to biochemical recurrence (BCR). BCR was defined as a PSA ≥ 0.2 ng/mL or treatment with radiation or androgen-deprivation therapy for a rising PSA < 0.2 ng/mL.
Results
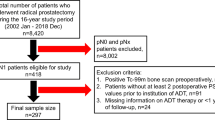
Overall, 72 patients were included of whom the majority had a Gleason score ≥ 8 (n = 46, 63.9%). Following neoadjuvant therapy, 55.7% of patients (n = 39/70) had pT3 disease, 40% (n = 28) had seminal vesicle invasion, 12.9% (n = 9) had positive margins, and 11.4% (n = 8) had lymph node involvement. Overall, 11 (15.7%) had tumor measuring ≤ 0.5 cm, which included four patients (5.7%) with a pathologic complete response and seven (10.0%) with residual tumor measuring 0.1–0.5 cm. Compared to pretreatment clinical staging, 10 patients (14.3%) had pathologic T downstaging at RP. The median follow-up was 3.4 years. Overall, the 3-year BCR-free rate was 70% (95% CI 57%, 90%). Of the 15 patients with either residual tumor ≤ 0.5 cm or pathologic T downstaging, no patient experienced a recurrence.
Conclusion
In this exploratory pooled clinical trials analysis, we highlight that neoadjuvant therapy prior to RP in unfavorable intermediate and high-risk patients may potentially have a positive impact on recurrence rates. Larger studies with longer follow-up periods are warranted to evaluate the impact of neoadjuvant hormone therapy on pathologic and long-term outcomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Eggener SE, Scardino PT, Walsh PC, Han M, Partin AW, Trock BJ, et al. Predicting 15-year prostate cancer specific mortality after radical prostatectomy. J Urol. 2011;185:869–75.
Brockman JA, Alanee S, Vickers AJ, Scardino PT, Wood DP, Kibel AS, et al. Nomogram predicting prostate cancer-specific mortality for men with biochemical recurrence after radical prostatectomy. Eur Urol. 2015;67:1160–7.
Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:188–94.
Meeks JJ, Bellmunt J, Bochner BH, Clarke NW, Daneshmand S, Galsky MD, et al. A systematic review of neoadjuvant and adjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol. 2012;62:523–33.
Advanced Bladder Cancer Meta-analysis C. Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis. Lancet. 2003;361:1927–34.
Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12:681–92.
Kidane B, Coughlin S, Vogt K, Malthaner R. Preoperative chemotherapy for resectable thoracic esophageal cancer. Cochrane Database Syst Rev. 2015:CD001556. Issue 5.
McKay RR, Choueiri TK, Taplin ME. Rationale for and review of neoadjuvant therapy prior to radical prostatectomy for patients with high-risk prostate cancer. Drugs. 2013;73:1417–30.
Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32.
Peintinger F, Sinn B, Hatzis C, Albarracin C, Downs-Kelly E, Morkowski J, et al. Reproducibility of residual cancer burden for prognostic assessment of breast cancer after neoadjuvant chemotherapy. Mod Pathol. 2015;28:913–20.
Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972;22:232–40.
Yee DS, Lowrance WT, Eastham JA, Maschino AC, Cronin AM, Rabbani F. Long-term follow-up of 3-month neoadjuvant hormone therapy before radical prostatectomy in a randomized trial. BJU Int. 2010;105:185–90.
van der Kwast TH, Tetu B, Candas B, Gomez JL, Cusan L, Labrie F. Prolonged neoadjuvant combined androgen blockade leads to a further reduction of prostatic tumor volume: three versus six months of endocrine therapy. Urology. 1999;53:523–9.
Soloway MS, Pareek K, Sharifi R, Wajsman Z, McLeod D, Wood DP Jr., et al. Neoadjuvant androgen ablation before radical prostatectomy in cT2bNxMo prostate cancer: 5-year results. J Urol. 2002;167:112–6.
Selli C, Montironi R, Bono A, Pagano F, Zattoni F, Manganelli A, et al. Effects of complete androgen blockade for 12 and 24 weeks on the pathological stage and resection margin status of prostate cancer. J Clin Pathol. 2002;55:508–13.
Schulman CC, Debruyne FM, Forster G, Selvaggi FP, Zlotta AR, Witjes WP. 4-Year follow-up results of a European prospective randomized study on neoadjuvant hormonal therapy prior to radical prostatectomy in T2-3N0M0 prostate cancer. European Study Group on Neoadjuvant Treatment of Prostate Cancer. Eur Urol. 2000;38:706–13.
Prezioso D, Lotti T, Polito M, Montironi R. Neoadjuvant hormone treatment with leuprolide acetate depot 3.75mg and cyproterone acetate, before radical prostatectomy: a randomized study. Urol Int. 2004;72:189–95.
Labrie F, Cusan L, Gomez JL, Diamond P, Suburu R, Lemay M, et al. Neoadjuvant hormonal therapy: the Canadian experience. Urology. 1997;49:56–64.
Klotz LH, Goldenberg SL, Jewett MA, Fradet Y, Nam R, Barkin J, et al. Long-term followup of a randomized trial of 0 versus 3 months of neoadjuvant androgen ablation before radical prostatectomy. J Urol. 2003;170:791–4.
Gleave ME, Goldenberg SL, Chin JL, Warner J, Saad F, Klotz LH, et al. Randomized comparative study of 3 versus 8-month neoadjuvant hormonal therapy before radical prostatectomy: biochemical and pathological effects. J Urol. 2001;166:500–6. discussion6-7
Fair WR, Rabbani F, Bastar A, Betancourt J. Neoadjuvant hormone therapy before radical prostatectomy: update on the Memorial Sloan-Kettering Cancer Center Trials. Mol Urol. 1999;3:253–60.
Dalkin BL, Ahmann FR, Nagle R, Johnson CS. Randomized study of neoadjuvant testicular androgen ablation therapy before radical prostatectomy in men with clinically localized prostate cancer. J Urol. 1996;155:1357–60.
Aus G, Abrahamsson PA, Ahlgren G, Hugosson J, Lundberg S, Schain M, et al. Three-month neoadjuvant hormonal therapy before radical prostatectomy: a 7-year follow-up of a randomized controlled trial. BJU Int. 2002;90:561–6.
Shelley MD, Kumar S, Wilt T, Staffurth J, Coles B, Mason MD. A systematic review and meta-analysis of randomised trials of neo-adjuvant hormone therapy for localised and locally advanced prostate carcinoma. Cancer Treat Rev. 2009;35:9–17.
Mostaghel EA, Page ST, Lin DW, Fazli L, Coleman IM, True LD, et al. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: therapeutic implications for castration-resistant prostate cancer. Cancer Res. 2007;67:5033–41.
Taplin ME, Montgomery B, Logothetis CJ, Bubley GJ, Richie JP, Dalkin BL, et al. Intense androgen-deprivation therapy with abiraterone acetate plus leuprolide acetate in patients with localized high-risk prostate cancer: results of a randomized phase II neoadjuvant study. J Clin. Oncol. 2014;32:3705–15.
Mostaghel EA, Nelson PS, Lange P, Lin DW, Taplin ME, Balk S, et al. Targeted androgen pathway suppression in localized prostate cancer: a pilot study. J Clin. Oncol. 2014;32:229–37.
Montgomery B, Tretiakova MS, Joshua AM, Gleave ME, Fleshner N, Bubley GJ et al. Neoadjuvant enzalutamide prior to prostatectomy. Clin Cancer Res. 2017;23:2169-2176.
James ND, de Bono JS, Spears MR, Clarke NW, Mason MD, Dearnaley DP, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377:338–51.
Tosoian JJ, Gorin MA, Ross AE, Pienta KJ, Tran PT, Schaeffer EM. Oligometastatic prostate cancer: definitions, clinical outcomes, and treatment considerations. Nat Rev Urol. 2017;14:15–25.
Temple LK, Hsieh L, Wong WD, Saltz L, Schrag D. Use of surgery among elderly patients with stage IV colorectal cancer. J Clin Oncol 2004;22:3475–84.
Mickisch GH, Garin A, van Poppel H, de Prijck L, Sylvester R, European Organisation for R et al. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet. 2001;358:966–70.
Culp SH, Schellhammer PF, Williams MB. Might men diagnosed with metastatic prostate cancer benefit from definitive treatment of the primary tumor? A SEER-based study. Eur Urol. 2014;65:1058–66.
Pierorazio PM, Gorin MA, Ross AE, Feng Z, Trock BJ, Schaeffer EM, et al. Pathological and oncologic outcomes for men with positive lymph nodes at radical prostatectomy: The Johns Hopkins Hospital 30-year experience. Prostate. 2013;73:1673–80.
McKay RR, Zurita AJ, Werner L, Bruce JY, Carducci MA, Stein MN, et al. A Randomized Phase II trial of short-course androgen deprivation therapy with or without bevacizumab for patients with recurrent prostate cancer after definitive local therapy. J Clin Oncol. 2016;34:1913–20.
Acknowledgements
We acknowledge the patients and families who participated in trials included in this analysis.
Funding
This work was supported by Janssen, Medivation, Prostate Cancer Foundation, A. David Mazzone Award Program, and the Fairweather Family Fund. The funding sponsors were not involved in the design of study, data collection and analysis, and manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
RRM received research funding from Bayer and Pfizer. QDT is supported by an unrestricted educational grant from the Vattikuti Urology Institute, a Clay Hamlin Young Investigator Award from the Prostate Cancer Foundation and a Genentech BioOncology Career Development Award from the Conquer Cancer Foundation of the American Society of Clinical Oncology. RBM received research support from Jansen, Medivation and ESSA. ASK receives advisory board honorarium from Janssen, Bayer, and Sanofi-Aventis. MET receives research funding and advisory board honorarium from Janssen, Medivation.
Rights and permissions
About this article
Cite this article
McKay, R.R., Montgomery, B., Xie, W. et al. Post prostatectomy outcomes of patients with high-risk prostate cancer treated with neoadjuvant androgen blockade. Prostate Cancer Prostatic Dis 21, 364–372 (2018). https://doi.org/10.1038/s41391-017-0009-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-017-0009-6
This article is cited by
-
Shifting the paradigm in high-risk prostate cancer: how good is TNM alone?
Prostate Cancer and Prostatic Diseases (2023)
-
Neoadjuvant radiohormonal therapy for oligo-metastatic prostate cancer: safety and efficacy outcomes from an open-label, dose-escalation, single-center, phase I/II clinical trial
Frontiers of Medicine (2023)
-
Neoadjuvant hormonal therapy before radical prostatectomy in high-risk prostate cancer
Nature Reviews Urology (2021)
-
Neoadjuvant treatment with androgen receptor signaling inhibitors prior to radical prostatectomy: a systematic review
World Journal of Urology (2021)
-
Surgical management of high-risk, localized prostate cancer
Nature Reviews Urology (2020)