Abstract
Background
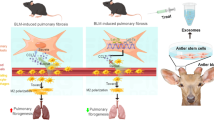
Pulmonary fibrosis (PF) is a progressive lung disorder with a high mortality rate; its therapy remains limited due to the inefficiency of drug delivery. In this study, the system of drug delivery of nintedanib (Nin) by exosomes derived from adipose-derived stem cells (ADSCs-Exo, Exo) was developed to effectively deliver Nin to lung lesion tissue to ensure enhanced anti-fibrosis therapy.
Methods
The bleomycin (BLM)-induced PF model was constructed in vivo and in vitro. The effects of Exo-Nin on BLM-induced PF and its regulatory mechanism were examined using RT-qPCR, Western blotting, immunofluorescence, and H&E staining.
Results
We found Exo-Nin significantly improved BLM-induced PF in vivo and in vitro compared to Nin and Exo groups alone. Mechanistically, Exo-Nin alleviated fibrogenesis by suppressing endothelial–mesenchymal transition through the down-regulation of the TGF-β/Smad pathway and the attenuation of oxidative stress in vivo and in vitro.
Conclusions
Utilizing adipose stem cell-derived exosomes as carriers for Nin exhibited a notable enhancement in therapeutic efficacy. This improvement can be attributed to the regenerative properties of exosomes, indicating promising prospects for adipose-derived exosomes in cell-free therapies for PF.
Impact
-
The system of drug delivery of nintedanib (Nin) by exosomes derived from adipose-derived stem cells was developed to effectively deliver Nin to lung lesion tissue to ensure enhanced anti-fibrosis therapy.
-
The use of adipose stem cell-derived exosomes as the carrier of Nin may increase the therapeutic effect of Nin, which can be due to the regenerative properties of the exosomes and indicate promising prospects for adipose-derived exosomes in cell-free therapies for PF.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Richeldi, L., Collard, H. & Jones, M. Idiopathic pulmonary fibrosis. Lancet 389, 1941–1952 (2017).
Wynn, T. Cellular and molecular mechanisms of fibrosis. J. Pathol. 214, 199–210 (2008).
Wynn, T. & Ramalingam, T. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat. Med. 18, 1028–1040 (2012).
Hutchinson, J., Fogarty, A., Hubbard, R. & McKeever, T. Global incidence and mortality of idiopathic pulmonary fibrosis: a systematic review. Eur. Respir. J. 46, 795–806 (2015).
Somogyi, V. et al. The therapy of idiopathic pulmonary fibrosis: what is next? Eur. Respir. Rev. 28, 190021 (2019).
Zhang, Z. et al. Crossed pathways for radiation-induced and immunotherapy-related lung injury. Fronti. Immunol. 12, 774807 (2021).
McPherson, M., Economidou, S., Liampas, A., Zis, P. & Parperis, K. Management of MDA-5 antibody positive clinically amyopathic dermatomyositis associated interstitial lung disease: a systematic review. Semin. Arthritis Rheum. 53, 151959 (2022).
Mohammadalipour, A., Hashemnia, M., Goudarzi, F. & Ravan, A. Increasing the effectiveness of tyrosine kinase inhibitor (TKI) in combination with a statin in reducing liver fibrosis. Clin. Exp. Pharmacol. Physiol. 46, 1183–1193 (2019).
Neul, C. et al. Impact of membrane drug transporters on resistance to small-molecule tyrosine kinase inhibitors. Trends Pharmacol. Sci. 37, 904–932 (2016).
Pan, L. et al. Nintedanib ameliorates bleomycin-induced pulmonary fibrosis, inflammation, apoptosis, and oxidative stress by modulating PI3K/Akt/mTOR pathway in mice. Inflammation 46, 1531–1542 (2023).
Ntolios, P. et al. Feasibility and safety of treatment switch from Pirfenidone to Nintedanib in patients with idiopathic pulmonary fibrosis: a real-world observational study. Eur. Rev. Med. Pharmacol. Sci. 25, 6326–6332 (2021).
Jiang, L. et al. Engineering exosomes endowed with targeted delivery of triptolide for malignant melanoma therapy. ACS Appl. Mater. Interfaces 13, 42411–42428 (2021).
Ashour, A., El-Kamel, A., Mehanna, R., Mourad, G. & Heikal, L. Luteolin-loaded exosomes derived from bone marrow mesenchymal stem cells: a promising therapy for liver fibrosis. Drug Deliv. 29, 3270–3280 (2022).
Qu, M. et al. Dopamine-loaded blood exosomes targeted to brain for better treatment of Parkinson’s disease. J. Controlled Release 287, 156–166 (2018).
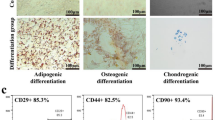
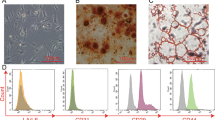
Bunnell, B. Adipose tissue-derived mesenchymal stem cells. Cells 10, 3433 (2021).
Li, J. et al. Paracrine factors from mesenchymal stem cells: a proposed therapeutic tool for acute lung injury and acute respiratory distress syndrome. Int. Wound J. 11, 114–121 (2014).
Chen, L. et al. Mesenchymal stem cell-based treatments for COVID-19: status and future perspectives for clinical applications. Cell. Mol. Life Sci. 79, 142 (2022).
Wang, X. et al. Exosomes from adipose-derived mesenchymal stem cells alleviate sepsis-induced lung injury in mice by inhibiting the secretion of IL-27 in macrophages. Cell Death Discov. 8, 18 (2022).
Guy, R. & Offen, D. Promising opportunities for treating neurodegenerative diseases with mesenchymal stem cell-derived exosomes. Biomolecules 10, 1320 (2020).
Shen, W., Zhao, X. & Li, S. Exosomes derived from ADSCs attenuate sepsis-induced lung injury by delivery of Circ-Fryl and regulation of the miR-490-3p/SIRT3 pathway. Inflammation 45, 331–342 (2022).
Estornut, C., Milara, J., Bayarri, M., Belhadj, N. & Cortijo, J. Targeting oxidative stress as a therapeutic approach for idiopathic pulmonary fibrosis. Front. Pharmacol. 12, 794997 (2021).
van der Vliet, A., Janssen-Heininger, Y. & Anathy, V. Oxidative stress in chronic lung disease: from mitochondrial dysfunction to dysregulated redox signaling. Mol. Aspects Med. 63, 59–69 (2018).
Hecker, L. et al. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat. Med. 15, 1077–1081 (2009).
Kilic, T., Ciftci, O., Cetin, A. & Kahraman, H. Preventive effect of chrysin on bleomycin-induced lung fibrosis in rats. Inflammation 37, 2116–2124 (2014).
Wolters, P., Collard, H. & Jones, K. Pathogenesis of idiopathic pulmonary fibrosis. Annu. Rev. Pathol. 9, 157–179 (2014).
Gao, Z. et al. Berberine-loaded M2 macrophage-derived exosomes for spinal cord injury therapy. Acta Biomater. 126, 211–223 (2021).
Yu, W. et al. Nintedanib inhibits endothelial mesenchymal transition in bleomycin-induced pulmonary fibrosis via focal adhesion kinase activity reduction. Int. J. Mol. Sci. 23, 8193 (2022).
Wollin, L. et al. Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur. Respir. J. 45, 1434–1445 (2015).
Matute-Bello, G. et al. Fas (CD95) induces alveolar epithelial cell apoptosis in vivo: implications for acute pulmonary inflammation. Am. J. Pathol. 158, 153–161 (2001).
Li, S., Zhou, Y., Gu, X., Zhang, X. & Jia, Z. NLRX1/FUNDC1/NIPSNAP1-2 axis regulates mitophagy and alleviates intestinal ischaemia/reperfusion injury. Cell Prolif. 54, e12986 (2021).
Lv, H. et al. Xanthohumol ameliorates lipopolysaccharide (LPS)-induced acute lung injury via induction of AMPK/GSK3β-Nrf2 signal axis. Redox Biol. 12, 311–324 (2017).
Kheirollahi, V. et al. Metformin induces lipogenic differentiation in myofibroblasts to reverse lung fibrosis. Nat. Commun. 10, 2987 (2019).
Yang, Z. et al. Inhibition of Wnt10b/β-catenin signaling alleviates pulmonary fibrogenesis induced by paraquat in vivo and in vitro. Life Sci. 286, 120027 (2021).
Mirsaidi, A. et al. Telomere length, telomerase activity and osteogenic differentiation are maintained in adipose-derived stromal cells from senile osteoporotic SAMP6 mice. J. Tissue Eng. Regen. Med. 6, 378–390 (2012).
Fatima, F. & Nawaz, M. Stem cell-derived exosomes: roles in stromal remodeling, tumor progression, and cancer immunotherapy. Chin. J. Cancer 34, 541–553 (2015).
Jandl, K., Radic, N., Zeder, K., Kovacs, G. & Kwapiszewska, G. Pulmonary vascular fibrosis in pulmonary hypertension - the role of the extracellular matrix as a therapeutic target. Pharmacol. Ther. 247, 108438 (2023).
Almuqbil, R. et al. Dendrimer conjugation enhances tumor penetration and efficacy of doxorubicin in extracellular matrix-expressing 3D lung cancer models. Mol. Pharm. 17, 1648–1662 (2020).
Farooqi, A. et al. Exosome biogenesis, bioactivities and functions as new delivery systems of natural compounds. Biotechnol. Adv. 36, 328–334 (2018).
Kooijmans, S., Vader, P. & Schiffelers, R. Tumour-bound RNA-laden exosomes. Nat. Biomed. Eng. 1, 634–636 (2017).
Sheykhhasan, M. et al. Neuroprotective effects of coenzyme Q10-loaded exosomes obtained from adipose-derived stem cells in a rat model of Alzheimer’s disease. Biomed. Pharmacother. 152, 113224 (2022).
Wei, Z. et al. Exosomes for gene therapy effectively inhibit the endothelial-mesenchymal transition in mouse aortic endothelial cells. BMC Musculoskelet. Disord. 22, 1000 (2021).
Mei, Q., Liu, Z., Zuo, H., Yang, Z. & Qu, J. Idiopathic pulmonary fibrosis: an update on pathogenesis. Front. Pharmacol. 12, 797292 (2021).
Yang, W. et al. Nintedanib alleviates pulmonary fibrosis in vitro and in vivo by inhibiting the FAK/ERK/S100A4 signalling pathway. Int. Immunopharmacol. 113, 109409 (2022).
C, L. et al. Common molecular pathways targeted by nintedanib in cancer and IPF: a bioinformatic study. Pulm. Pharmacol. Ther. 64, 101941 (2020).
Fernandez, I. & Eickelberg, O. The impact of TGF-β on lung fibrosis: from targeting to biomarkers. Proc. Am. Thorac. Soc. 9, 111–116 (2012).
Louzada, R. et al. NADPH oxidase DUOX1 sustains TGF-β1 signalling and promotes lung fibrosis. Eur. Respir. J. 57, 1901949 (2021).
Meng, X., Nikolic-Paterson, D. & Lan, H. TGF-β: the master regulator of fibrosis. Nat. Rev. Nephrol. 12, 325–338 (2016).
Otoupalova, E., Smith, S., Cheng, G. & Thannickal, V. Oxidative stress in pulmonary fibrosis. Compr. Physiol. 10, 509–547 (2020).
Divya, T., Dineshbabu, V., Soumyakrishnan, S., Sureshkumar, A. & Sudhandiran, G. Celastrol enhances Nrf2 mediated antioxidant enzymes and exhibits anti-fibrotic effect through regulation of collagen production against bleomycin-induced pulmonary fibrosis. Chem. Biol. Interact. 246, 52–62 (2016).
Zhang, Z. et al. Nrf2 antioxidant pathway suppresses Numb-mediated epithelial-mesenchymal transition during pulmonary fibrosis. Cell Death Dis. 9, 83 (2018).
Huai, B. & Ding, J. Atractylenolide III attenuates bleomycin-induced experimental pulmonary fibrosis and oxidative stress in rat model via Nrf2/NQO1/HO-1 pathway activation. Immunopharmacol. Immunotoxicol. 42, 436–444 (2020).
Acknowledgements
The authors gratefully acknowledge the financial supports by the Educational Commission of Fujian Province (No. JAT200702), as well as the Xiamen Medical College (No. K2020-05).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cai, L., Wang, J., Yi, X. et al. Nintedanib-loaded exosomes from adipose-derived stem cells inhibit pulmonary fibrosis induced by bleomycin. Pediatr Res (2024). https://doi.org/10.1038/s41390-024-03024-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-024-03024-7