Abstract
Background
Pediatric Post-COVID-Condition (PPCC) clinics treat children despite limited scientific substantiation. By exploring real-life management of children diagnosed with PPCC, the International Post-COVID-Condition in Children Collaboration (IP4C) aimed to provide guidance for future PPCC care.
Methods
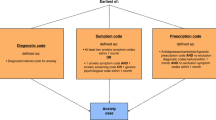
We performed a cross-sectional international, multicenter study on used PPCC definitions; the organization of PPCC care programs and patients characteristics. We compared aggregated data from PPCC cohorts and identified priorities to improve PPCC care.
Results
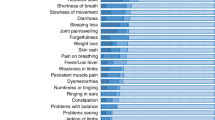
Ten PPCC care programs and six COVID-19 follow-up research cohorts participated. Aggregated data from 584 PPCC patients was analyzed. The most common symptoms included fatigue (71%), headache (55%), concentration difficulties (53%), and brain fog (48%). Severe limitations in daily life were reported in 31% of patients. Most PPCC care programs organized in-person visits with multidisciplinary teams. Diagnostic testing for respiratory and cardiac morbidity was most frequently performed and seldom abnormal. Treatment was often limited to physical therapy and psychological support.
Conclusions
We found substantial heterogeneity in both the diagnostics and management of PPCC, possibly explained by scarce scientific evidence and lack of standardized care. We present a list of components which future guidelines should address, and outline priorities concerning PPCC care pathways, research and international collaboration.
Impact
-
Pediatric Post-COVID Condition (PPCC) Care programs have been initiated in many countries.
-
Children with PPCC in different countries are affected by similar symptoms, limiting many to participate in daily life.
-
There is substantial heterogeneity in diagnostic testing. Access to specific diagnostic tests is required to identify some long-term COVID-19 sequelae. Treatments provided were limited to physical therapy and psychological support.
-
This study emphasizes the need for evidence-based diagnostics and treatment of PPCC. The International Post-COVID Collaboration for Children (IP4C) provides guidance for guideline development and introduces a framework of priorities for PPCC care and research, to improve PPCC outcomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
References
Brackel, C. L. H. et al. Pediatric long-COVID: an overlooked phenomenon? Pediatr. Pulmonol. 56, 2495–2502 (2021).
Ashkenazi-Hoffnung, L. et al. Long COVID in children: observations from a designated pediatric clinic. Pediatr. Infect. Dis. J. 40, e509–e511 (2021).
O’Mahoney, L. et al. The prevalence and long-term health effects of Long COVID among hospitalized and non-hospitalized populations: a systematic review and meta-analysis. eClinicalMedicine 55, 101762 (2023).
Global Burden of Disease Long COVID Collaborators. Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA 328, 1604–1615 (2022).
Zimmermann, P., Pittet, L. F. & Curtis, N. How common is long COVID in children and adolescents? Pediatr. Infect. Dis. J. 40, e482–e487 (2021).
Morrow, A. K. et al. Postacute/long COVID in pediatrics: development of a multidisciplinary rehabilitation clinic and preliminary case series. Am. J. Phys. Med. Rehabil. 100, 1140–1147 (2021).
WHO. Clinical Management of COVID-19: Living Guideline, 13 January 2023 (World Health Organization, 2023).
Buonsenso, D. It is OK to tell patients with long COVID that we don’t know much about the condition yet. Acta Paediatr. 122, 585–6 (2023).
Davis, H. E., McCorkell, L., Vogel, J. M. & Topol, E. J. Long COVID: major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 21, 133–146 (2023).
Feedback on the Long Covid Clinics. Preliminary healthcare experiences survey findings (longcovidkids.org).
Buonsenso, D. et al. Evidence of lung perfusion defects and ongoing inflammation in an adolescent with post-acute sequelae of SARS-CoV-2 infection. Lancet Child Adolesc. Health 5, 677–680 (2021).
Seylanova, N. et al. Core outcome measurement set for research and clinical practice in post COVID-19 condition (Long COVID) in children and young people: an International Delphi Consensus Study ‘PC-COS Children’. Eur. Respir. J. (2024).
Malone, L. A. et al. Multi-disciplinary collaborative consensus guidance statement on the assessment and treatment of postacute sequelae of SARS-CoV-2 infection (PASC) in children and adolescents. PM R 14, 1241–1269 (2022).
Buonsenso, D. et al. Long-term outcomes of pediatric infections: from traditional infectious diseases to long COVID. Future Microbiol. 17, 551–571 (2022).
Wacks, M., Wortley, E., Gregorowski, A., Segal, T. Y. & Whittaker, E. Fifteen-minute consultation: managing post-COVID-19 syndrome (long COVID) in children and young people. Arch. Dis. Child. Educ. Pract. Ed. https://doi.org/10.1136/archdischild-2022-324950 (2023).
Clinical management of COVID-19: living guideline, 18 August 2023. Geneva: World Health Organization; 2023 (WHO/2019-nCoV/clinical/2023.2). Licence: CC BY-NC-SA 3.0 IGO.
WHO. https://www.who/int/europe/publication/i/item/WHO-EURO-2023-8018-47786-70552 (2023).
Rajan, S. et al. In the Wake of the Pandemic: Preparing for Long COVID (Policy Brief 39) (European Observatory on Health Systems and Policies, 2021).
Funding
T.S., S.P.P., R.S. and M.d.N. were funded by the Department of Health and Social Care, in their capacity as the National Institute for Health Research (NIHR), and by UK Research & Innovation (UKRI), who have awarded funding grant number COVLT0022. S.P.P. is also supported by a UK Medical Research Council Career Development Award (ref: MR/P020372/1). The department of Health and Social Care, as the NIHR, and URI were not involved in study design, data collection, analysis or interpretation of the data, nor the writing of the present study or the decision to submit the article for publication. All research at Great Ormond Street Hospital NHS Foundation Trust and UCL Great Ormond Street Institute of Child Health is made possible by the NIHR Great Ormond Street Hospital Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, UKRI, or the Department of Health. All other authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
C.L.H.B. and L.C.E.N. conceptualized and designed the study, designed the data collection instruments, collected data, carried out the initial analyses, drafted the initial manuscript and reviewed and revised the manuscript. C.L.L., S.J.H.V., A.H.M.-v.d.Z., J.B.v.G., S.H. and S.W.J.T.-L. conceptualized and designed the study, critically reviewed the analyses, and reviewed and revised the manuscript. D.B., D.M., L.S., L.A., L.A.-H., A.E., O.H., M.L., L.A.M., M.M., D.W.M., A.K.M., C.R.O., E.P., M.R.-R., R.S., T.S., S.T., L.V., and D.V. conceptualized the study, reviewed the data collection tools, collected data and reviewed the manuscript. A.P.N.B., N.L.S.D., T.F., R.G.J., A.L.N.G., E.H., V.H., L.N.J., N.K., R.K., P.K., S.M., M.D.N., P.R.S.O., I.M.O., I.B.O., S.P.P., Y.P., N.D.P., R.C.F.R., M.R., C.D.R., E.S., T.S.J., D.S., J.T.S., I.S.-L., E.S., A.S., N.T., M.T., P.V., and L.R.S.V. collected data and critically reviewed the manuscript. All authors contributed to the framework, approved the final manuscript as submitted and agree to be accountable for all aspects of the manuscript.
Corresponding author
Ethics declarations
Competing interests
L.S. received support by the UK Foreign, Commonwealth and Development Office and Wellcome [215091/Z/18/Z] and the Bill & Melinda Gates Foundation [OPP1209135]. C.R.O. and E.P. were supported, in part, from grants by the National Institutes of Health (NIH) to C.R.O. (K23AI159518), its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIH. T.S. is Chair of the Health Research Authority. All other authors have no conflicts of interest relevant to this article to disclose.
Ethical approval and consent to participate
This study was performed in line with the principles of the Declaration of Helsinki. Patient consent was not required, due to the use of aggregated and fully anonymized data. Approvement was granted by the medical research ethics committee of the Amsterdam University Medical Centers, location AMC, which evaluated this project as exempt from the Medical Research Involving Human Subjects Act (WMO) (W21_550#21.606). The study was approved by each center’s institutional review board.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brackel, C.L.H., Noij, L.C.E., Vijverberg, S.J.H. et al. International Care programs for Pediatric Post-COVID Condition (Long COVID) and the way forward. Pediatr Res (2024). https://doi.org/10.1038/s41390-023-03015-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-023-03015-0
This article is cited by
-
Clinical-based phenotypes in children with pediatric post-COVID-19 condition
World Journal of Pediatrics (2024)