Abstract
Background
Large-for-gestational age (LGA), a marker of fetal overgrowth, has been linked to obesity in adulthood. Little is known about how infancy growth trajectories affect adiposity in early childhood in LGA.
Methods
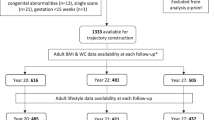
In the Shanghai Birth Cohort, we followed up 259 LGA (birth weight >90th percentile) and 1673 appropriate-for-gestational age (AGA, 10th–90th percentiles) children on body composition (by InBody 770) at age 4 years. Adiposity outcomes include body fat mass (BFM), percent body fat (PBF), body mass index (BMI), overweight/obesity, and high adiposity (PBF >85th percentile).
Results
Three weight growth trajectories (low, mid, and high) during infancy (0–2 years) were identified in AGA and LGA subjects separately. BFM, PBF and BMI were progressively higher from low- to mid-to high-growth trajectories in both AGA and LGA children. Compared to the mid-growth trajectory, the high-growth trajectory was associated with greater increases in BFM and the odds of overweight/obesity or high adiposity in LGA than in AGA children (tests for interactions, all P < 0.05).
Conclusions
Weight trajectories during infancy affect adiposity in early childhood regardless of LGA or not. The study is the first to demonstrate that high-growth weight trajectory during infancy has a greater impact on adiposity in early childhood in LGA than in AGA subjects.
Impact
-
Large-for-gestational age (LGA), a marker of fetal overgrowth, has been linked to obesity in adulthood, but little is known about how weight trajectories during infancy affect adiposity during early childhood in LGA subjects.
-
The study is the first to demonstrate a greater impact of high-growth weight trajectory during infancy (0–2 years) on adiposity in early childhood (at age 4 years) in subjects with fetal overgrowth (LGA) than in those with normal birth size (appropriate-for-gestational age).
-
Weight trajectory monitoring may be a valuable tool in identifying high-risk LGA children for close follow-ups and interventions to decrease the risk of obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
As access to the deidentified participant research data must be approved by the research ethics board on a case-by-case basis, please contact the corresponding authors (zc.luo@utoronto.ca; feili@shsmu.edu.cn; srachel@126.com) for assistance in data access request.
References
Ng, M. et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384, 766–781 (2014).
Abarca-Gómez, L. et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 390, 2627–2642 (2017).
Falkner, B. & Gidding, S. Life-course implications of pediatric risk factors for cardiovascular disease. Can. J. Cardiol. 37, 766–775 (2021).
Marcus, C., Danielsson, P. & Hagman, E. Pediatric obesity-long-term consequences and effect of weight loss. J. Intern. Med. 292, 870–891 (2022).
Bocca-Tjeertes, I. F. et al. Growth patterns of large for gestational age children up to age 4 years. Pediatrics 133, e643–e649 (2014).
Harvey, L., van Elburg, R. & van der Beek, E. M. Macrosomia and large for gestational age in Asia: one size does not fit all. J. Obstet. Gynaecol. Res. 47, 1929–1945 (2021).
Palatianou, M. E., Simos, Y. V., Andronikou, S. K. & Kiortsis, D. N. Long-term metabolic effects of high birth weight: a critical review of the literature. Horm. Metab. Res. 46, 911–920 (2014).
Yu, Z. B. et al. Birth weight and subsequent risk of obesity: a systematic review and meta-analysis. Obes. Rev. 12, 525–542 (2011).
Johannsson, E., Arngrimsson, S. A., Thorsdottir, I. & Sveinsson, T. Tracking of overweight from early childhood to adolescence in cohorts born 1988 and 1994: overweight in a high birth weight population. Int. J. Obes. 30, 1265–1271 (2006).
Gu, S. et al. Risk factors and long-term health consequences of macrosomia: a prospective study in Jiangsu Province, China. J. Biomed. Res. 26, 235–240 (2012).
Woo, J. G. Infant growth and long-term cardiometabolic health: a review of recent findings. Curr. Nutr. Rep. 8, 29–41 (2019).
Corvalan, C., Kain, J., Weisstaub, G. & Uauy, R. Impact of growth patterns and early diet on obesity and cardiovascular risk factors in young children from developing countries. Proc. Nutr. Soc. 68, 327–337 (2009).
Zheng, M. et al. Rapid weight gain during infancy and subsequent adiposity: a systematic review and meta-analysis of evidence. Obes. Rev. 19, 321–332 (2018).
Gatjens, I. et al. Relationship between birth weight, early growth rate, and body composition in 5- to 7-year-old children. Obes. Facts 15, 519–527 (2022).
Gishti, O. et al. Fetal and infant growth patterns associated with total and abdominal fat distribution in school-age children. J. Clin. Endocrinol. Metab. 99, 2557–2566 (2014).
Shi, H. et al. Insights into infancy weight gain patterns for term small-for-gestational-age babies. Nutr. J. 17, 1–9 (2018).
Norris, T. et al. Early childhood weight gain: latent patterns and body composition outcomes. Paediatr. Perinat. Epidemiol. 35, 557–568 (2021).
Matsumoto, N. et al. Trajectory of body mass index and height changes from childhood to adolescence: a nationwide birth cohort in Japan. Sci. Rep. 11, 1–10 (2021).
Lei, X. et al. Childhood health outcomes in term, large-for-gestational-age babies with different postnatal growth patterns. Am. J. Epidemiol. 187, 507–514 (2018).
Zhang, J. et al. Cohort profile: the Shanghai Birth Cohort. Int. J. Epidemiol. 48, 1–8 (2019).
Zhu, L. et al. [Chinese neonatal birth weight curve for different gestational age]. Chin. J. Pediatr. 53, 97–103 (2015).
Li, H., Ji, C., Zong, X. & Zhang, Y. [Body mass index growth curves for Chinese children and adolescents aged 0 to 18 years]. Chin. J. Pediatr. 47, 493–498 (2009).
Tasdelen, B., Ozge, A., Kaleagasi, H., Erdogan, S. & Mengi, T. Determining of migraine prognosis using latent growth mixture models. Chin. Med. J. 124, 1044–1049 (2011).
Arrandale, V., Koehoorn, M., MacNab, Y. & Kennedy, S. M. How to use SAS® Proc Traj and SAS® Proc Glimmix in respiratory epidemiology. https://open.library.ubc.ca/media/stream/pdf/52383/1.0048205/1 (2006).
van Buuren, S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat. Methods Med. Res. 16, 219–242 (2007).
Hill, D. J. et al. Relationship between birth weight and metabolic status in obese adolescents. ISRN Obes. 2013, 1–8 (2013).
Olander, R. F. W., Sundholm, J. K. M., Suonsyrja, S. & Sarkola, T. Arterial health during early childhood following abnormal fetal growth. BMC Pediatr. 22, 1–12 (2022).
Honda, M. et al. Birth weight was associated positively with gluteofemoral fat mass and inversely with 2-h postglucose insulin concentrations, a marker of insulin resistance, in young normal-weight Japanese women. Diabetol. Int. 13, 375–380 (2022).
Derraik, J. G. B. et al. Large-for-gestational-age phenotypes and obesity risk in adulthood: a study of 195,936 women. Sci. Rep. 10, 1–9 (2020).
Santos-Silva, R. et al. Persistent weight gain between 0 and 4 years of age is associated with higher dehydroepiandrosterone sulphate levels at 7 years old: data from the Generation XXI birth cohort. Clin. Endocrinol. 97, 588–595 (2022).
Taal, H. R., Vd Heijden, A. J., Steegers, E. A., Hofman, A. & Jaddoe, V. W. Small and large size for gestational age at birth, infant growth, and childhood overweight. Obesity 21, 1261–1268 (2013).
Salgin, B. et al. Even transient rapid infancy weight gain is associated with higher BMI in young adults and earlier menarche. Int. J. Obes. 39, 939–944 (2015).
Ekelund, U. et al. Upward weight percentile crossing in infancy and early childhood independently predicts fat mass in young adults: the Stockholm Weight Development Study (SWEDES). Am. J. Clin. Nutr. 83, 324–330 (2006).
Samaranayake, D. et al. Association between early weight gain and later adiposity in Sri Lankan adolescents. J. Dev. Orig. Health Dis. 12, 250–259 (2021).
Druet, C. et al. Prediction of childhood obesity by infancy weight gain: an individual-level meta-analysis. Paediatr. Perinat. Epidemiol. 26, 19–26 (2012).
Acknowledgements
We gratefully acknowledged all research staff who had contributed to patient recruitment and data collection in the Shanghai Birth Cohort.
Funding
This study was supported by grants from the Ministry of Science and Technology of China (2019YFA0802501), National Natural Science Foundation of China (81930095, 82125032, 82204064, 81903323, 81803244, 81761128035), the Shanghai Science and Technology Commission (19410713500 and 2018SHZDZX01), the Shanghai Municipal Health Commission (GWV-10.1-XK07, 2020CXJQ01, 2018YJRC03), the Shanghai Clinical Key Subject Construction Project (shslczdzk02902), the Shanghai Pujiang Program (22PJD045), and the Canadian Institutes of Health Research (158616). The funders have no role in all aspects of the study, including study design, data collection and analysis, the preparation of the manuscript and the decision for publication.
Author information
Authors and Affiliations
Consortia
Contributions
Z.-C.L., F.L., X.-H.S., J.Z., and F.O. conceived the study. M.-Y.T., X.L., Z.-L.C., M.-N.Y., Y.-J.X., H.H., F.F., Q.C., X.-X.M., J.Z., F.O., X.-H.S., F.L., and Z.-C.L. contributed to the acquisition of research data. M.-Y.T. and X.L. conducted the literature review, data analysis, and drafted the manuscript. All authors contributed in revising the article critically for important intellectual content, and approved the final version for publication. Z.-C.L. is the guarantor who takes full responsibility for the work as a whole.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics
The study was approved by the research ethics committees of Xinhua Hospital (the coordination center, reference number M2013-010) and all participating hospitals. Written informed consent was obtained from all study participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tao, MY., Liu, X., Chen, ZL. et al. Fetal overgrowth and weight trajectories during infancy and adiposity in early childhood. Pediatr Res 95, 1372–1378 (2024). https://doi.org/10.1038/s41390-023-02991-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02991-7