Abstract
Background
Neonatal Sepsis accounts for significant proportion of neonatal mortality globally. Ciprofloxacin can be used as an effective antimicrobial against common causative agents of neonatal sepsis. However, there is only limited information about its pharmacokinetic distribution in plasma and Cerebrospinal fluid (CSF) of neonates.
Methods
Plasma and CSF samples were taken using a sparse sampling technique from neonates who received at least one dose of intravenous ciprofloxacin. Ciprofloxacin levels were analysed using high-performance liquid chromatography (HPLC). Population pharmacokinetic analysis was conducted using a non-linear mixed-effects modelling using Pumas® (Pharmaceutical Modelling and Simulation) package (Version 2.0).
Results
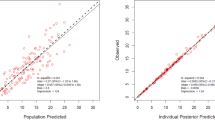
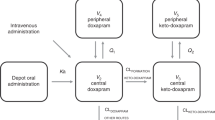
53 neonates were enroled in the study of whom; 9 (17%) had meningitis. The median concentration of ciprofloxacin in CSF was 1.4 (0.94–2.06) ug/ml and plasma was 2.94 (1.8–5.0) ug/ml. A one-compartment model with first-order elimination fitted the data. Body weight was found to be a significant covariate on volume of distribution (Vd). Simulations based on the final model suggest that dose of 10 mg/kg, intravenous b.d may not be able to achieve the desirable indices.
Conclusions
One compartment model with weight as a covariate explained the available data. Further studies with modified sampling strategy, larger sample size and variable dose levels are needed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Liu, L. et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet 388, 3027–3035 (2016).
Ku, L. C., Boggess, K. A. & Cohen-Wolkowiez, M. Bacterial meningitis in infants. Clin. Perinatol. 42, 29–45 (2015).
Furyk, J. S., Swann, O. & Molyneux, E. Systematic review: neonatal meningitis in the developing world. Trop. Med Int Health 16, 672–679 (2011).
Cantey, J. B. & Milstone, A. M. Bloodstream infections: epidemiology and resistance. Clin. Perinatol. 42, 1–16 (2015).
Anderson, V., Anderson, P., Grimwood, K. & Nolan, T. Cognitive and executive function 12 years after childhood bacterial meningitis: Effect of acute neurologic complications and age of onset. J. Pediatr. Psychol. 29, 67–81 (2004).
Evans-Roberts, K. M. et al. DNA gyrase is the target for the quinolone drug ciprofloxacin in Arabidopsis thaliana. J. Biol. Chem. 291, 3136–3144 (2016).
Sharma, D. et al. Interplay of the quality of ciprofloxacin and antibiotic resistance in developing countries. Front Pharm. 8, 546 (2017).
Tebano, G. et al. Essential and forgotten antibiotics: An inventory in low- and middle-income countries. Int. J. Antimicrob. Agents 54, 273–282 (2019).
Nau, R., Sorgel, F. & Eiffert, H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin. Microbiol Rev. 23, 858–883 (2010).
Nau, R. et al. Penetration of ciprofloxacin into the cerebrospinal fluid of patients with uninflamed meninges. J. Antimicrob. Chemother. 25, 965–973 (1990).
Wolff, M. et al. Penetration of ciprofloxacin into cerebrospinal fluid of patients with bacterial meningitis. Antimicrob. Agents Chemother. 31, 899–902 (1987).
Lipman, J. et al. Ciprofloxacin pharmacokinetic profiles in paediatric sepsis: how much ciprofloxacin is enough? Intensive Care Med. 28, 493–500 (2002).
Rubio, T. T. et al. Pharmacokinetic disposition of sequential intravenous/oral ciprofloxacin in pediatric cystic fibrosis patients with acute pulmonary exacerbation. Pediatr. Infect. Dis. J. 16, 112–117 (1997).
Dr Veronica Zanichelli, McMaster University. 2021 WHO Expert Committee on Selection and Use of Essential Medicines 2021. Available from: https://cdn.who.int/media/docs/default-source/essential-medicines/2021-eml-expert-committee/applications-for-new-indications-for-existing-listed-medicines/i.6_ab-neonatal-meningitis.pdf?sfvrsn=db6508d6_5.
Chaudhari, S., Suryawanshi, P., Ambardekar, S., Chinchwadkar, M. & Kinare, A. Safety profile of ciprofloxacin used for neonatal septicemia. Indian Pediatr. 41, 1246–1251 (2004).
Dutta, S., Chowdhary, G., Kumar, P., Mukhopadhay, K. & Narang, A. Ciprofloxacin administration to very low birth weight babies has no effect on linear growth in infancy. J. Trop. Pediatr. 52, 103–106 (2006).
National Institute for Health and Care Excellance. Neonatal infection: antibiotics for prevention and treatment (2021). [Available from: https://www.nice.org.uk/guidance/ng195.
Zimmermann, P. & Curtis, N. Normal values for cerebrospinal fluid in neonates: A systematic review. Neonatology 118, 629–638 (2021).
Muchohi, S. N. et al. Determination of ciprofloxacin in human plasma using high-performance liquid chromatography coupled with fluorescence detection: application to a population pharmacokinetics study in children with severe malnutrition. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 879, 146–152 (2011).
Zhao, W. et al. Population pharmacokinetics of ciprofloxacin in neonates and young infants less than three months of age. Antimicrob. Agents Chemother. 58, 6572–6580 (2014).
Baudry-Simner, P. J. et al. Mechanisms of reduced susceptibility to ciprofloxacin in Escherichia coli isolates from Canadian hospitals. Can. J. Infect. Dis. Med Microbiol 23, e60–e64 (2012).
Aggarwal, P., Dutta, S., Garg, S. K. & Narang, A. Multiple dose pharmacokinetics of ciprofloxacin in preterm babies. Indian Pediatr. 41, 1001–1007 (2004).
Kaguelidou, F., Turner, M. A., Choonara, I. & Jacqz-Aigrain, E. Ciprofloxacin use in neonates: a systematic review of the literature. Pediatr. Infect. Dis. J. 30, e29–e37 (2011).
Hirt, D. et al. Population pharmacokinetics of intravenous and oral ciprofloxacin in children to optimize dosing regimens. Eur. J. Clin. Pharm. 77, 1687–1695 (2021).
Pea, F., Viale, P., Pavan, F. & Furlanut, M. Pharmacokinetic considerations for antimicrobial therapy in patients receiving renal replacement therapy. Clin. Pharmacokinet. 46, 997–1038 (2007).
Facchin, A. et al. Variability of ciprofloxacin pharmacokinetics in children: impact on dose range in sickle cell patients. J. Antimicrob. Chemother. 73, 3423–3429 (2018).
Author information
Authors and Affiliations
Contributions
K.G. for writing the initial draft, RKB for analysis and re-review of manuscript along with N.S., N.S. for conceptualizing designing, planning and manuscript writing; pharmacokinetic modelling and preparation of manuscript; S.K.M. for data analysis and critical review of manuscript; D.C. for data analysis and critical review of manuscript; S.J. for biochemical sample separation and storage, M.S.R. for giving expert view on pharmacokinetic modelling; S.K. for conceptualisation of study, writing, data analysis and critical review of manuscript; J.D.B. for assisting in pharmacokinetic modelling.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Informed consent
Consent was taken from either of parent’s before enroling neonate in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Garg, K., Bhandari, R.K., Shafiq, N. et al. Population pharmacokinetics of ciprofloxacin in newborns with early onset neonatal sepsis and suspected meningitis. Pediatr Res 95, 1273–1278 (2024). https://doi.org/10.1038/s41390-023-02941-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02941-3