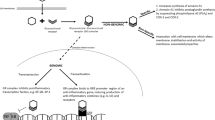
Abstract
Background
Lung inflammation and impaired alveolarization precede bronchopulmonary dysplasia (BPD). Glucocorticoids are anti-inflammatory and reduce ventilator requirements in preterm infants. However, high-dose glucocorticoids inhibit alveolarization. The effect of glucocorticoids on lung function and structure in preterm newborns exposed to antenatal inflammation is unknown. We hypothesise that postnatal low-dose dexamethasone reduces ventilator requirements, prevents inflammation and BPD-like lung pathology, following antenatal inflammation.
Methods
Pregnant ewes received intra-amniotic LPS (E.coli, 4 mg/mL) or saline at 126 days gestation; preterm lambs were delivered 48 h later. Lambs were randomised to receive either tapered intravenous dexamethasone (LPS/Dex, n = 9) or saline (LPS/Sal, n = 10; Sal/Sal, n = 9) commencing <3 h after birth. Respiratory support was gradually de-escalated, using a standardised protocol aimed at weaning from ventilation towards unassisted respiration. Tissues were collected at day 7.
Results
Lung morphology and mRNA levels for inflammatory mediators were measured. Respiratory support requirements were not different between groups. Histological analyses revealed higher tissue content and unchanged alveolarization in LPS/Sal compared to other groups. LPS/Dex lambs exhibited decreased markers of pulmonary inflammation compared to LPS/Sal.
Conclusion
Tapered low-dose dexamethasone reduces the impact of antenatal LPS on ventilation requirements throughout the first week of life and reduces inflammation and pathological thickening of the preterm lung
Impact
-
We are the first to investigate the combination of antenatal inflammation and postnatal dexamethasone therapy in a pragmatic study design, akin to contemporary neonatal care. We show that antenatal inflammation with postnatal dexamethasone therapy does not reduce ventilator requirements, but has beneficial maturational impacts on the lungs of preterm lambs at 7 days of life. Appropriate tapered postnatal dexamethasone dosing should be explored for extuabtion of oxygen-dependant neonates.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this article and its supplementary material files. Further enquiries can be directed to the corresponding author.
References
Stoll, B. J. et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA 314, 1039–1051 (2015).
Kair, L. R., Leonard, D. T. & Anderson, J. M. Bronchopulmonary dysplasia. Pediatr. Rev. 33, 255–263 (2012).
Lahra, M. M., Beeby, P. J. & Jeffery, H. E. Maternal versus fetal inflammation and respiratory distress syndrome: A 10-year hospital cohort study. Arch. Dis. Child. Fetal Neonatal Ed. 94, F13–F16 (2009).
Jobe, A. The new bpd. NeoReviews 7, e531–e545 (2006).
Doyle, L. W., Ehrenkranz, R. A. & Halliday, H. L. Early (< 8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst. Rev. CD001146 (2014); update in Cochrane Database Syst. Rev. 10, CD001146 (2017).
Barrington, K. J. The adverse neuro-developmental effects of postnatal steroids in the preterm infant: A systematic review of rcts. BMC Pediatr. 1, 1 (2001).
Cummings, J. J., D’Eugenio, D. B. & Gross, S. J. A controlled trial of dexamethasone in preterm infants at high risk for bronchopulmonary dysplasia. N. Engl. J. Med. 320, 1505–1510 (1989).
Gupta, S., Prasanth, K., Chen, C. M. & Yeh, T. F. Postnatal corticosteroids for prevention and treatment of chronic lung disease in the preterm newborn. Int J. Pediatr. 2012, 315642 (2012).
Newborn CoFa. Postnatal corticosteroids to treat or prevent chronic lung disease in preterm infants. Paediatr. Child Health 7, 20–46 (2002).
Doyle, L. W. et al. Low-dose dexamethasone facilitates extubation among chronically ventilator-dependent infants: A multicenter, international, randomized, controlled trial. Pediatrics 117, 75–83 (2006).
Husain, A. N., Siddiqui, N. H. & Stocker, J. T. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum. Pathol. 29, 710–717 (1998).
Agrons, G. A., Courtney, S. E., Stocker, J. T. & Markowitz, R. I. From the archives of the afip: Lung disease in premature neonates: Radiologic-pathologic correlation. Radiographics 25, 1047–1073 (2005).
National Health and Medical Research Council. Australian code for the care and use of animals for scientific purposes. 8th ed. Canberra: National Health and Medical Research Council; 2013.
Papagianis, P. C. et al. The effect of human amnion epithelial cells on lung development and inflammation in preterm lambs exposed to antenatal inflammation. PloS One 16, e0253456 (2021).
Kramer, B. W. et al. Dose and time response after intraamniotic endotoxin in preterm lambs. Am. J. Respir Crit. Care Med. 164, 982–988 (2001).
Willet, K. E. et al. Antenatal endotoxin and glucocorticoid effects on lung morphometry in preterm lambs. Pediatr. Res. 48, 782–788 (2000).
Joyce, B. J. et al. Sustained changes in lung expansion alter tropoelastin mrna levels and elastin content in fetal sheep lungs. Am. J. Physiol. Lung Cell. Mol. Physiol. 284, L643–L649 (2003).
Polglase, G. R. et al. Effects of antenatal melatonin therapy on lung structure in growth-restricted newborn lambs. J. Appl. Physiol. 123, 1195–1203 (2017).
Bischof, R. J., Snibson, K., Shaw, R. & Meeusen, E. N. Induction of allergic inflammation in the lungs of sensitized sheep after local challenge with house dust mite. Clin. Exp. Allergy 33, 367–375 (2003).
Inocencio, I. M. et al. Exacerbation of ventilation-induced lung injury and inflammation in preterm lambs by high-dose nanoparticles. Sci. Rep. 7, 14704 (2017).
Allison, B. J. et al. Ventilation of the very immature lung in utero induces injury and bpd-like changes in lung structure in fetal sheep. Pediatr. Res. 64, 387–392 (2008).
Medhurst, A. D. et al. The use of taqman rt-pcr assays for semiquantitative analysis of gene expression in cns tissues and disease models. J. Neurosci. Methods 98, 9–20 (2000).
Hellemans, J. et al. Qbase relative quantification framework and software for management and automated analysis of real-time quantitative pcr data. Genome Biol. 8, R19 (2007).
Vandesompele, J. et al. Accurate normalization of real-time quantitative rt-pcr data by geometric averaging of multiple internal control genes. Genome Biol. 3, RESEARCH0034 (2002).
Moss, T. J. et al. Early gestational intra-amniotic endotoxin: Lung function, surfactant, and morphometry. Am. J. Respir. Crit. Care Med. 165, 805–811 (2002).
Hillman, N. H. et al. Antenatal and postnatal corticosteroid and resuscitation induced lung injury in preterm sheep. Respir. Res. 10, 124 (2009).
Moss, T. J., Harding, R. & Newnham, J. P. Lung function, arterial pressure and growth in sheep during early postnatal life following single and repeated prenatal corticosteroid treatments. Early Hum. Dev. 66, 11–24 (2002).
Jobe, A. H., Newnham, J. P., Moss, T. J. & Ikegami, M. Differential effects of maternal betamethasone and cortisol on lung maturation and growth in fetal sheep. Am. J. Obstet. Gynecol. 188, 22–28 (2003).
Kuypers, E. et al. Intra-amniotic lps and antenatal betamethasone: Inflammation and maturation in preterm lamb lungs. Am. J. Physiol. Lung Cell. Mol. Physiol. 302, L380–L389 (2012).
Kuschel, C., Evans, N. & Lam, A. Prediction of individual response to postnatal dexamethasone in ventilator dependent preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 78, F199–F203 (1998).
Powell, K., Kerkering, K. W., Barker, G. & Rozycki, H. J. Dexamethasone dosing, mechanical ventilation and the risk of cerebral palsy. J. Matern.-Fetal Neonatal Med. : Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. 19, 43–48 (2006).
Zhou, J., Yu, Z. & Chen, C. Hydrocortisone for preventing mortality and bronchopulmonary dysplasia in preterm infants with or without chorioamnionitis exposure: A meta-analysis of randomized trials. Am. J. Perinatol. 38, 662–668 (2021).
Kaufman, D. & Fairchild, K. D. Clinical microbiology of bacterial and fungal sepsis in very-low-birth-weight infants. Clin. Microbiol. Rev. 17, 638–80, (2004).
Weber, M. A. et al. Infection and sudden unexpected death in infancy: A systematic retrospective case review. Lancet 371, 1848–1853 (2008).
Zhao, J., Gonzalez, F. & Mu, D. Apnea of prematurity: From cause to treatment. Eur. J. Pediatr. 170, 1097–1105 (2011).
Lorea-Hernandez, J. J. et al. Microglia modulate respiratory rhythm generation and autoresuscitation. Glia 64, 603–619 (2016).
Froen, J. F., Akre, H., Stray-Pedersen, B. & Saugstad, O. D. Adverse effects of nicotine and interleukin-1beta on autoresuscitation after apnea in piglets: Implications for sudden infant death syndrome. Pediatrics 105, E52 (2000).
Banat, G. A. et al. Immune and inflammatory cell composition of human lung cancer stroma. PloS One 10, e0139073 (2015).
Jobe, A., Mitchell, B. R. & Gunkel, H. Beneficial effects of the combined use of prenatal corticosteroids and postnatal surfactant on preterm infants. Am. J. Obstet. Gynecol. 168, 508–513 (1993).
Kramer, B. W. et al. Antenatal betamethasone changes cord blood monocyte responses to endotoxin in preterm lambs. Pediatr. Res. 55, 764–768 (2004).
Moss, T. J. et al. Effects into adulthood of single or repeated antenatal corticosteroids in sheep. Am. J. Obstet. Gynecol. 192, 146–152 (2005).
Polglase, G. R. et al. Maternal and intra-amniotic corticosteroid effects on lung morphometry in preterm lambs. Pediatr. Res. 62, 32–36 (2007).
Neubauer, K. et al. Decrease of pecam-1-gene-expression induced by proinflammatory cytokines ifn-gamma and ifn-alpha is reversed by tgf-beta in sinusoidal endothelial cells and hepatic mononuclear phagocytes. BMC Physiol. 8, 9 (2008).
Swaroop, J. & O’Reilly, R. A. Splenomegaly at a university hospital compared to a nearby county hospital in 317 patients. Acta Haematol. 102, 83–88 (1999).
Albertine, K. H. et al. Chronic lung injury in preterm lambs. Disordered respiratory tract development. Am. J. Respir. Crit. Care Med. 159, 945–958 (1999).
Funding
This research was supported by: NHMRC Project Grant (1057759); NHMRC Centre for Research Excellence (1057514); NHMRC Career Development Fellowship (Peter Noble, 1045824); 2 NHMRC Senior Research Fellowships (Moss 1043294; Pillow,1077691); Victorian Government’s Operational Infrastructure Support Program; West Australian Government’s Medical and Health Research Infrastructure Fund. Unrestricted equipment and consumable support was provided by Chiesi Farmaceutici S.p.A. (poractant alfa); Fisher & Paykel Healthcare (ventilator circuits); and ICU Medical (arterial monitoring lines).
Author information
Authors and Affiliations
Contributions
P.C.P. designed and performed tissue analyses, collected data, analysed data and drafted manuscript. S.A. and P.B.N. performed experiments. T.J.M. helped design and perform tissue analyses, contributed to the interpretation and presentation of data and drafted manuscript. J.J.P. conceived the study, was responsible for the study design, had overall responsibility for and supervised all aspects of lamb studies and drafted manuscript. J.J.P. and P.B.N. obtained funding for the study. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Jane Pillow has acted as a consultant on behalf of The University of Western Australia for Chiesi Farmaceutici S.p.A. on projects unrelated to the focus of the current manuscript.
Ethics
Study protocols were reviewed and approved by The University of Western Australia Animal Ethics Committee (RA-3/100/1301).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Papagianis, P.C., Noble, P.B., Ahmadi-Noorbakhsh, S. et al. Postnatal steroids as lung protective and anti-inflammatory in preterm lambs exposed to antenatal inflammation. Pediatr Res 95, 931–940 (2024). https://doi.org/10.1038/s41390-023-02911-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02911-9
This article is cited by
-
Early career insight
Pediatric Research (2024)
-
Is there a role for early postnatal steroids in very preterm infants exposed to chorioamnionitis?
Pediatric Research (2024)