Abstract
Background
Postnatal steroids are used to prevent bronchopulmonary dysplasia in extremely preterm infants but may have adverse effects on brain development. We assessed connectivity metrics of major cerebral and cerebellar white matter pathways at near-term gestational age among infants who did or did not receive a standardized regimen of hydrocortisone during the first 10 days of life.
Methods
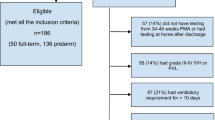
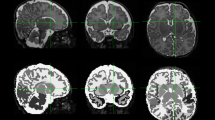
Retrospective cohort study. Participants: Infants born <28 weeks: Protocol group (n = 33) received at least 50% and not more than 150% of an intended standard dose of 0.5 mg/kg hydrocortisone twice daily for 7 days, then 0.5 mg/kg per day for 3 days; Non-Protocol group (n = 22), did not receive protocol hydrocortisone or completed <50% of the protocol dose. We assessed group differences in near-term diffusion MRI mean fractional anisotropy (FA) and mean diffusivity (MD) across the corticospinal tract, inferior longitudinal fasciculus, corpus callosum and superior cerebellar peduncle.
Results
Groups were comparable in gestational age, post-menstrual age at scan, medical complications, bronchopulmonary dysplasia, and necrotizing enterocolitis. No significant large effect group differences were identified in mean FA or MD in any cerebral or cerebellar tract.
Conclusion(s)
Low dose, early, postnatal hydrocortisone was not associated with significant differences in white matter tract microstructure at near-term gestational age.
Impact
-
This study compared brain microstructural connectivity as a primary outcome among extremely preterm infants who did or did not receive early postnatal hydrocortisone.
-
Low dose hydrocortisone in the first 10 days of life was not associated with significant differences in white matter microstructure in major cerebral and cerebellar pathways.
-
Hydrocortisone did not have a significant effect on early brain white matter circuits.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Deidentified data will be made available upon reasonable request made to the corresponding author.
References
Jobe, A. The new bronchopulmonary dysplasia. Curr. Opin. Pediatr. 23, 167–172 (2011).
Jobe, A. H. & Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 163, 1723–1729 (2001).
Volpe, J. J. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 8, 110–124 (2009).
Watterberg, K. L. & Scott, S. M. Evidence of early adrenal insufficiency in babies who develop bronchopulmonary dysplasia. Pediatrics 95, 120–125 (1995).
Doyle, L. W. & Anderson, P. J. Long-term outcomes of bronchopulmonary dysplasia. Semin. Fetal Neonatal Med. 14, 391–395 (2009).
Baud, O. et al. Effect of early low-dose hydrocortisone on survival without bronchopulmonary dysplasia in extremely preterm infants (PREMILOC): a double-blind, placebo-controlled, multicentre, randomized trial. Lancet 387, 1827–1836 (2016).
Alison, M. et al. Prophylactic hydrocortisone in extremely preterm infants and brain MRI abnormality. Arch. Dis. Child Fetal Neonatal Ed. 105, 520–525 (2020).
Baud, O. et al. Association between early low-dose hydrocortisone therapy in extremely preterm neonates and neurodevelopmental outcomes at 2 years of age. JAMA 317, 1329–1337 (2017).
Baud, O. et al. Two-year neurodevelopmental outcomes of extremely preterm infants treated with early hydrocortisone: treatment effect according to gestational age at birth. Arch. Dis. Child. Fetal Neonatal Ed. 104, F30–F35 (2019).
Thompson, D. K. et al. Corpus callosum alterations in very preterm infants: perinatal correlates and 2 year neurodevelopmental outcomes. Neuroimage 59, 3571–3581 (2012).
Shah, D. K. et al. Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J. Pediatr. 153, 170–5, 175.e1 (2008).
Volpe, J. J., Kinney, H. C., Jensen, F. E. & Rosenberg, P. A. The developing oligodendrocyte: key cellular target in brain injury in the premature infant. Int. J. Dev. Neurosci. 29, 423–440 (2011).
Chang, Y. P. Evidence for adverse effect of perinatal glucocorticoid use on the developing brain. Korean J. Pediatr. 57, 101–109 (2014).
Huang, W. L., Dunlop, S. A. & Harper, C. G. Effect of exogenous corticosteroids on the developing central nervous system: a review. Obstetrical Gynecol. Surv. 54, 336–342 (1999).
Noguchi, K. K. Glucocorticoid induced cerebellar toxicity in the developing neonate: implications for glucocorticoid therapy during bronchopulmonary dysplasia. Cells 3, 36–52 (2014).
Feldman, H. M., Yeatman, J. D., Lee, E. S., Barde, L. H. & Gaman-Bean, S. Diffusion tensor imaging: a review for pediatric researchers and clinicians. J. Dev. Behav. Pediatr. 31, 346–356 (2010).
Lerma-Usabiaga, G., Mukherjee, P., Perry, M. L. & Wandell, B. A. Data-science ready, multisite, human diffusion MRI white-matter-tract statistics. Sci. Data 7, 422 (2020).
Liu, M., Lerma-Usabiaga, G., Clascá, F. & Paz-Alonso, P. M. Reproducible protocol to obtain and measure first-order relay human thalamic white-matter tracts. Neuroimage 262, 119558 (2022).
Zöllei, L., Iglesias, J. E., Ou, Y., Grant, P. E. & Fischl, B. Infant FreeSurfer: an automated segmentation and surface extraction pipeline for T1-weighted neuroimaging data of infants 0-2 years. Neuroimage 218, 116946 (2020).
Blesa, M. et al. Parcellation of the healthy neonatal brain into 107 regions using atlas propagation through intermediate time points in childhood. Front. Neurosci. 10, 220 (2016).
Veraart, J., Fieremans, E. & Novikov, D. S. Diffusion MRI noise mapping using random matrix theory. Magn. Reson. Med. 76, 1582–1593 (2016).
Veraart, J. et al. Denoising of diffusion MRI using random matrix theory. Neuroimage 142, 394–406 (2016).
Kellner, E., Dhital, B., Kiselev, V. G. & Reisert, M. Gibbs-ringing artifact removal based on local subvoxel-shifts. Magn. Reson. Med. 76, 1574–1581 (2016).
Andersson, J. L. R. & Sotiropoulos, S. N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage 125, 1063–1078 (2016).
Yeatman, J. D., Dougherty, R. F., Myall, N. J., Wandell, B. A. & Feldman, H. M. Tract profiles of white matter properties: automating fiber-tract quantification. PloS One 7, e49790 (2012).
Tournier, J.-D., Calamante, F. & Connelly, A. Robust determination of the fibre orientation distribution in diffusion MRI: non-negativity constrained super-resolved spherical deconvolution. Neuroimage 35, 1459–1472 (2007).
Takemura, H., Caiafa, C. F., Wandell, B. A. & Pestilli, F. Ensemble tractography. PLoS Comput. Biol. 12, e1004692 (2016).
Tournier, J.-D. et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualization. Neuroimage 202, 116137 (2019).
Dougherty, R. F. et al. Temporal-callosal pathway diffusivity predicts phonological skills in children. Proc. Natl Acad. Sci. USA 104, 8556–8561 (2007).
Huang, H. et al. DTI tractography based parcellation of white matter: application to the mid-sagittal morphology of corpus callosum. Neuroimage 26, 195–205 (2005).
de Kieviet, J. F. et al. A crucial role of altered fractional anisotropy in motor problems of very preterm children. Eur. J. Paediatr. Neurol. 18, 126–133 (2014).
Kelly, C. E. et al. Brain structural and microstructural alterations associated with cerebral palsy and motor impairments in adolescents born extremely preterm and/or extremely low birthweight. Dev. Med. Child Neurol. 57, 1168–1175 (2015).
Dubner S. E., Rose J., Bruckert L., Feldman H. M. & Travis, K. E. Neonatal white matter tract microstructure and 2-year language outcomes after preterm birth. NeuroImage Clin. 28, 102446 (2020).
Feldman, H. M., Lee, E. S., Yeatman, J. D. & Yeom, K. W. Language and reading skills in school-aged children and adolescents born preterm are associated with white matter properties on diffusion tensor imaging. Neuropsychologia 50, 3348–3362 (2012).
Murner-Lavanchy, I. M. et al. White matter microstructure is associated with language in children born very preterm. Neuroimage Clin. 20, 808–822 (2018).
Back, S. A. Brain injury in the preterm infant: new horizons for pathogenesis and prevention. Pediatr. Neurol. 53, 185–192 (2015).
Dubner, S. E. et al. White matter microstructure and cognitive outcomes in relation to neonatal inflammation in 6-year-old children born preterm. NeuroImage: Clin. 101832 https://doi.org/10.1016/j.nicl.2019.101832 (2019).
Shim, S. Y. et al. Altered microstructure of white matter except the corpus callosum is independent of prematurity. Neonatology 102, 309–315 (2012).
Tam, E. W. Y. et al. Preterm cerebellar growth impairment after postnatal exposure to glucocorticoids. Sci. Transl. Med. 3, 105ra105 (2011).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 57, 289–300 (1995).
Faul, F., Erdfelder, E., Buchner, A. & Lang, A.-G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 41, 1149–1160 (2009).
Dibble, M., Ang, J. Z., Mariga, L., Molloy, E. J. & Bokde, A. L. W. Diffusion tensor imaging in very preterm, moderate-late preterm and term-born neonates: a systematic review. J. Pediatr. 232, 48–58.e3 (2021).
Rose, S. E. et al. Altered white matter diffusion anisotropy in normal and preterm infants at term-equivalent age. Magn. Reson. Med. 60, 761–767 (2008).
Benders, M. J. N. L. et al. Brain development of the preterm neonate after neonatal hydrocortisone treatment for chronic lung disease. Pediatr. Res. 66, 555–559 (2009).
Kersbergen, K. J. et al. Hydrocortisone treatment for bronchopulmonary dysplasia and brain volumes in preterm infants. J. Pediatr. 163, 666–671.e1 (2013).
Parikh, N., Kennedy, K., Lasky, R., McDavid, G. & Tyson, J. Pilot randomized trial of hydrocortisone in ventilator-dependent extremely preterm infants: effects on regional brain volumes. J. Pediatr. 162, 685–690.e1 (2013).
Rousseau, C., Guichard, M., Saliba, E., Morel, B. & Favrais, G. Duration of mechanical ventilation is more critical for brain growth than postnatal hydrocortisone in extremely preterm infants. Eur. J. Pediatr. https://doi.org/10.1007/s00431-021-04113-z (2021).
Bruckert, L. et al. Age-dependent white matter characteristics of the cerebellar peduncles from infancy through adolescence. Cerebellum 18, 372–387 (2019).
Yeatman, J. D., Wandell, B. A. & Mezer, A. A. Lifespan maturation and degeneration of human brain white matter. Nat. Commun. 5, 4932 (2014).
Barres, B. A., Lazar, M. A. & Raff, M. C. A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development 120, 1097–1108 (1994).
Watterberg, K. L. et al. Hydrocortisone to improve survival without bronchopulmonary dysplasia. N. Engl. J. Med. 386, 1121–1131 (2022).
Onland, W. et al. Effect of hydrocortisone therapy initiated 7 to 14 days after birth on mortality or bronchopulmonary dysplasia among very preterm infants receiving mechanical ventilation: a randomized clinical trial. JAMA 321, 354–363 (2019).
Naseh, N. et al. Early Hyperglycemia in very preterm infants is associated with reduced white matter volume and worse cognitive and motor outcomes at 2.5 years. Neonatology 1–8 https://doi.org/10.1159/000524923 (2022).
Funding
All phases of this study were supported by the Society for Developmental Behavioral Pediatrics Research Grant to S.E.D., and by the National Institutes of Health- Eunice Kennedy Shriver National Institute of Child Health and Human Development (K.E.T., PI; 5R00HD8474904; H.M.F., PI; 2RO1- HD069150). Role of Funder/Sponsor (if any): The NIH and SDBP had no role in the design and conduct of the study.
Author information
Authors and Affiliations
Contributions
S.E.D. conceptualized and designed the study, coordinated and supervised data collection, carried out the data analyses and interpretation, drafted the initial manuscript, and critically reviewed and revised the manuscript. L.R. collected data, performed initial data analyses, drafted the initial manuscript, and critically reviewed and revised the manuscript. D.S. collected data and critically reviewed and revised the manuscript. R.V.P. performed data analysis and critically reviewed and revised the manuscript. L.B. supervised and performed data analysis and critically reviewed and revised the manuscript. M.S. critically reviewed and revised the manuscript for important intellectual content. H.M.F. conceptualized and designed the study and critically reviewed and revised the manuscript for important intellectual content. K.E.T coordinated and supervised data collection, supervised analysis, and critically reviewed and revised the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dubner, S.E., Rickerich, L., Bruckert, L. et al. Early, low-dose hydrocortisone and near-term brain connectivity in extremely preterm infants. Pediatr Res 95, 1028–1034 (2024). https://doi.org/10.1038/s41390-023-02903-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02903-9