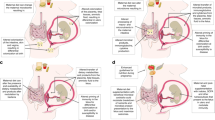
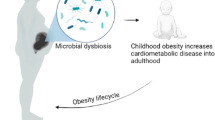
Abstract
While pregnancy post-bariatric surgery has become increasingly common, little is known about whether and how maternal bariatric surgery affects the next generation. This scoping review aimed to collate available evidence about the long-term health of offspring following maternal bariatric surgery. A literature search was conducted using three databases (PubMed, PsycINFO, EMBASE) to obtain relevant human and animal studies. A total of 26 studies were included: 17 were ancillary reports from five “primary” studies (three human, two animal studies) and the remaining nine were “independent” studies (eight human, one animal studies). The human studies adopted sibling-comparison, case-control, and single-group descriptive designs. Despite limited data and inconsistent results across studies, findings suggested that maternal bariatric surgery appeared to (1) modify epigenetics (especially genes involved in immune, glucose, and obesity regulation); (2) alter weight status (unclear direction of alteration); (3) impair cardiometabolic, immune, inflammatory, and appetite regulation markers (primarily based on animal studies); and (4) not affect the neurodevelopment in offspring. In conclusion, this review supports that maternal bariatric surgery has an effect on the health of offspring. However, the scarcity of studies and heterogenous findings highlight that more research is required to determine the scope and degree of such effects.
Impact
-
There is evidence that bariatric surgery modifies epigenetics in offspring, especially genes involved in immune, glucose, and obesity regulation.
-
Bariatric surgery appears to alter weight status in offspring, although the direction of alteration is unclear.
-
There is preliminary evidence that bariatric surgery impairs offspring’s cardiometabolic, immune, inflammatory, and appetite regulation markers. Therefore, extra care may be needed to ensure optimal growth in children born to mothers with previous bariatric surgery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Stierman, B. et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. National Health Statistics Reports. no. 158 (2021).
Sanchez, C. E. et al. Maternal pre-pregnancy obesity and child neurodevelopmental outcomes: a meta-analysis. Obes. Rev. 19, 464–484 (2018).
Lutsiv, O., Mah, J., Beyene, J. & McDonald, S. D. The effects of morbid obesity on maternal and neonatal health outcomes: a systematic review and meta-analyses. Obes. Rev. 16, 531–546 (2015).
Wu, H., Liu, F., Zhao, M., Liang, Y. & Xi, B. Maternal body mass index and risks of neonatal mortality and offspring overweight and obesity: findings from 0.5 million samples in 61 low- and middle-income countries. Pediatr. Obes. 15, e12665 (2020).
Kong, L., Nilsson, I. A. K., Brismar, K., Gissler, M. & Lavebratt, C. Associations of different types of maternal diabetes and body mass index with offspring psychiatric disorders. JAMA Netw. Open 3, e1920787 (2020).
Miras, A. D. & le Roux, C. W. Mechanisms underlying weight loss after bariatric surgery. Nat. Rev. Gastroenterol. Hepatol. 10, 575–584 (2013).
Welbourn, R. et al. Bariatric surgery worldwide: baseline demographic description and one-year outcomes from the Fourth IFSO Global Registry Report 2018. Obes. Surg. 29, 782–795 (2019).
American Society for Metabolic & Bariatric Surgery. Estimate of bariatric surgery numbers, 2011–2020, accessed 12/5/2022. https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers.
Youssefzadeh, A. C. et al. Pregnancy characteristics and outcomes after bariatric surgery: national-level analysis in the United States. Surg. Obes. Relat. Dis. 19, 364–373 (2023).
Khosravi-Largani, M. et al. Evaluation of all types of metabolic bariatric surgery and its consequences: a systematic review and meta-analysis. Obes. Surg. 29, 651–690 (2019).
Liu, D. F. et al. The effects of bariatric surgery on dyslipidemia and insulin resistance in overweight patients with or without type 2 diabetes: a systematic review and network meta-analysis. Surg. Obes. Relat. Dis. 17, 1655–1672 (2021).
Feichtinger, M. et al. Intrauterine fetal growth delay during late pregnancy after maternal gastric bypass surgery. Ultraschall Med. 41, 52–59 (2020).
Youssefzadeh, A. C. et al. Cesarean delivery after bariatric surgery: trends and outcomes in the United States. AJOG 226, S341 (2022).
Różańska-Walędziak, A. et al. The influence of bariatric surgery on pregnancy and perinatal outcomes-A case-control study. J. Clin. Med. 9, 1324 (2020).
Akhter, Z. et al. Pregnancy after bariatric surgery and adverse perinatal outcomes: a systematic review and meta-analysis. PLoS Med. 16, e1002866 (2019).
Johansson, K. et al. Outcomes of pregnancy after bariatric surgery. NEJM 372, 814–824 (2015).
Gascoin, G. et al. Risk of low birth weight and micronutrient deficiencies in neonates from mothers after gastric bypass: a case control study. Surg. Obes. Relat. Dis. 13, 1384–1391 (2017).
Adsit, J. & Hewlings, S. J. Impact of bariatric surgery on breastfeeding: a systematic review. Surg. Obes. Relat. Dis. 18, 117–122 (2022).
Arksey, H. & O’Malley, L. Scoping studies: towards a methodological framework. Int J. Soc. Res Methodol. 8, 19–32 (2005).
Munn, Z. et al. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 18, 143 (2018).
Spann, R. A., Taylor, E. B., Welch, B. A. & Grayson, B. E. Altered immune system in offspring of rat maternal vertical sleeve gastrectomy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 317, R852–r863 (2019).
Deer, E. M. et al. Dysregulated appetitive leptin signaling in male rodent offspring from post-bariatric dams. Curr. Res Physiol. 3, 50–58 (2020).
Spann, R. A., Welch, B. A. & Grayson, B. E. Ghrelin signalling is dysregulated in male but not female offspring in a rat model of maternal vertical sleeve gastrectomy. J. Neuroendocrinol. 33, e12913 (2021).
Grayson, B. E., Schneider, K. M., Woods, S. C. & Seeley, R. J. Improved rodent maternal metabolism but reduced intrauterine growth after vertical sleeve gastrectomy. Sci. Transl. Med. 5, 199ra112 (2013).
Ceglarek, V. M. et al. Maternal Roux-en-Y gastric bypass surgery reduces lipid deposition and increases UCP1 expression in the brown adipose tissue of male offspring. Sci. Rep. 11, 1158 (2021).
Pietrobon, C. B. et al. Maternal Roux-en-Y gastric bypass impairs insulin action and endocrine pancreatic function in male F1 offspring. Eur. J. Nutr. 59, 1067–1079 (2020).
Bertasso, I. M. et al. Pregnancy and lactation after Roux-en-Y gastric bypass worsen nonalcoholic fatty liver disease in obese rats and lead to differential programming of hepatic de novo lipogenesis in offspring. J. Dev. Orig. Health Dis. 13, 263–273 (2022).
Smith, J. et al. Effects of maternal surgical weight loss in mothers on intergenerational transmission of obesity. J. Clin. Endocrinol. Metab. 94, 4275–4283 (2009).
Kral, J. G. et al. Large maternal weight loss from obesity surgery prevents transmission of obesity to children who were followed for 2 to 18 years. Pediatrics 118, e1644–e1649 (2006).
Guénard, F. et al. Methylation and expression of immune and inflammatory genes in the offspring of bariatric bypass surgery patients. J. Obes. 2013, 492170 (2013).
Guénard, F. et al. Differential methylation in glucoregulatory genes of offspring born before vs. after maternal gastrointestinal bypass surgery. PNAS 110, 11439–11444 (2013).
Van De Maele, K., Devlieger, R., De Schepper, J. & Gies, I. Endothelial function and its determinants in children born after maternal bariatric surgery. Pediatr. Res. 91, 699–704 (2022).
Van De Maele, K. et al. Adiposity, psychomotor and behaviour outcomes of children born after maternal bariatric surgery. Pediatr. Obes. 16, e12749 (2021).
Van De Maele, K., De Geyter, C., Vandenplas, Y., Gies, I. & Devlieger, R. Eating habits of children born after maternal bariatric surgery. Nutrients 12, 2577 (2020).
Berglind, D. et al. Differential methylation in inflammation and type 2 diabetes genes in siblings born before and after maternal bariatric surgery. Obesity (Silver Spring) 24, 250–261 (2016).
Willmer, M. et al. Surgically induced interpregnancy weight loss and prevalence of overweight and obesity in offspring. PLoS One 8, e82247 (2013).
Berglind, D. et al. Differences in gestational weight gain between pregnancies before and after maternal bariatric surgery correlate with differences in birth weight but not with scores on the body mass index in early childhood. Pediatr. Obes. 9, 427–434 (2014).
Malik, S. et al. Maternal and fetal outcomes of Asian pregnancies after bariatric surgery. Surg. Obes. Relat. Dis. 16, 529–535 (2020).
Barisione, M., Carlini, F., Gradaschi, R., Camerini, G. & Adami, G. F. Body weight at developmental age in siblings born to mothers before and after surgically induced weight loss. Surg. Obes. Relat. Dis. 8, 387–391 (2012).
Dell’Agnolo, C. M., Cyr, C., de Montigny, F., de Barros Carvalho, M. D. & Pelloso, S. M. Pregnancy after bariatric surgery: obstetric and perinatal outcomes and the growth and development of children. Obes. Surg. 25, 2030–2039 (2015).
Larsson, L., Landin-Olsson, M. & Nilsson, C. Weight development in children after gastric bypass surgery. J. Fam. Reprod. Health 13, 176–180 (2019).
Del Sordo, G. et al. Postnatal health in children born to women after bariatric surgery. Obes. Surg. 30, 3898–3904 (2020).
Damti, P., Friger, M., Landau, D., Sergienko, R. & Sheiner, E. Offspring of women following bariatric surgery and those of patients with obesity are at an increased risk for long-term pediatric endocrine morbidity. Arch. Gynecol. Obstet. 300, 1253–1259 (2019).
Blume, C. A. et al. Association of maternal Roux-en-Y gastric bypass with obstetric outcomes and fluid intelligence in offspring. Obes. Surg. 28, 3611–3620 (2018).
Gimenes, J. C. et al. Nutritional status of children from women with previously bariatric surgery. Obes. Surg. 28, 990–995 (2018).
Kuhn, C., Covatti, C., Ribeiro, L. F. C., Balbo, S. L. & Torrejais, M. M. Bariatric surgery induces morphological changes in the extensor digitorum longus muscle in the offspring of obese rats. Tissue Cell 72, 101537 (2021).
Benton, M. C. et al. An analysis of DNA methylation in human adipose tissue reveals differential modification of obesity genes before and after gastric bypass and weight loss. Genome Biol. 16, 8 (2015).
Fraszczyk, E. et al. The effects of bariatric surgery on clinical profile, DNA methylation, and ageing in severely obese patients. Clin. Epigenetics 12, 14 (2020).
Nilsson, E. K. et al. Roux-en Y gastric bypass surgery induces genome-wide promoter-specific changes in DNA methylation in whole blood of obese patients. PLoS One 10, e0115186 (2015).
Garcia, L. A. et al. Weight loss after Roux-En-Y gastric bypass surgery reveals skeletal muscle DNA methylation changes. Clin. Epigenetics 13, 100 (2021).
Pinhel, M. A. S. et al. Changes in DNA methylation and gene expression of insulin and obesity-related gene PIK3R1 after Roux-en-Y gastric bypass. Int J. Mol. Sci. 21, 4476 (2020).
Wilhelm, J. et al. Promoter methylation of LEP and LEPR before and after bariatric surgery: a cross-sectional study. Obes. Facts 14, 1–7 (2021).
Wei, Y. et al. Enriched environment-induced maternal weight loss reprograms metabolic gene expression in mouse offspring. J. Biol. Chem. 290, 4604–4619 (2015).
Nicholas, L. M. et al. Differential effects of maternal obesity and weight loss in the periconceptional period on the epigenetic regulation of hepatic insulin-signaling pathways in the offspring. FASEB J. 27, 3786–3796 (2013).
Zhang, S. et al. Periconceptional undernutrition in normal and overweight ewes leads to increased adrenal growth and epigenetic changes in adrenal IGF2/H19 gene in offspring. FASEB J. 24, 2772–2782 (2010).
DiPietro, J. A. & Voegtline, K. M. The gestational foundation of sex differences in development and vulnerability. Neuroscience 342, 4–20 (2017).
Powell, T. L. et al. Sex-specific responses in placental fatty acid oxidation, esterification and transfer capacity to maternal obesity. Biochim Biophys. Acta Mol. Cell Biol. Lipids 1866, 158861 (2021).
Wijenayake, S. et al. Maternal high-fat diet induces sex-specific changes to glucocorticoid and inflammatory signaling in response to corticosterone and lipopolysaccharide challenge in adult rat offspring. J. Neuroinflamm. 17, 116 (2020).
Pradeilles, R. et al. Factors associated with catch-up growth in early infancy in rural Pakistan: a longitudinal analysis of the women’s work and nutrition study. Matern Child Nutr. 15, e12733 (2019).
den Harink, T. et al. Preconception lifestyle intervention in women with obesity and echocardiographic indices of cardiovascular health in their children. Int J. Obes. (Lond.) 46, 1262–1270 (2022).
Goedegebuure, W. J., Van der Steen, M., Smeets, C. C. J., Kerkhof, G. F. & Hokken-Koelega, A. C. S. SGA-born adults with postnatal catch-up have a persistently unfavourable metabolic health profile and increased adiposity at age 32 years. Eur. J. Endocrinol. 187, 15–26 (2022).
Martin, A., Connelly, A., Bland, R. M., Reilly, J. J. Health impact of catch-up growth in low-birth weight infants: systematic review, evidence appraisal, and meta-analysis. Matern Child Nutr. 13, https://doi.org/10.1111/mcn.12297 (2017).
Lee, D. et al. Glycemic patterns are distinct in post-bariatric hypoglycemia after gastric bypass (PBH-RYGB). J. Clin. Endocrinol. Metab. 106, 2291–2303 (2021).
Hanaire, H. et al. High glycemic variability assessed by continuous glucose monitoring after surgical treatment of obesity by gastric bypass. Diabetes Technol. Ther. 13, 625–630 (2011).
Capoccia, D. et al. Is type 2 diabetes really resolved after laparoscopic sleeve gastrectomy? Glucose variability studied by continuous glucose monitoring. J. Diabetes Res. 2015, 674268 (2015).
Borzì, A. M. et al. Endothelial function in obese patients treated with bariatric surgery. Diabetes Metab. Syndr. Obes. 13, 247–256 (2020).
Cerreto, M., Santopaolo, F., Gasbarrini, A., Pompili, M. & Ponziani, F. R. Bariatric surgery and liver disease: general considerations and role of the gut-liver axis. Nutrients 13, 2649 (2021).
Lutz, T. A. & Bueter, M. The use of rat and mouse models in bariatric surgery experiments. Front Nutr. 3, 25 (2016).
Spann, R. A. et al. Rodent vertical sleeve gastrectomy alters maternal immune health and fetoplacental development. Clin. Sci. (Lond.) 132, 295–312 (2018).
Himel, A. R., Cabral, S. A., Shaffery, J. P. & Grayson, B. E. Anxiety behavior and hypothalamic-pituitary-adrenal axis altered in a female rat model of vertical sleeve gastrectomy. PLoS One 13, e0200026 (2018).
Funding
V.L. was supported by the National Institute of Health, Building Interdisciplinary Research Careers in Women’s Health at UC Davis through Grant Number: 5K12HD051958.
Author information
Authors and Affiliations
Contributions
Y.Y.: conception and design; literature search and review; interpretation of results; drafting the article; final approval. V.L.: interpretation of results; revising the article; final approval. S.W.G.: interpretation of results; revising the article; final approval
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, Y., Lyo, V. & Groth, S.W. The impact of maternal bariatric surgery on long-term health of offspring: a scoping review. Pediatr Res 94, 1619–1630 (2023). https://doi.org/10.1038/s41390-023-02698-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02698-9