Abstract
Background
Extremely low birth weight (ELBW) infants comprise a fragile population at risk for neurodevelopmental disabilities (NDD). Systemic steroids were previously associated with NDD, but more recent studies suggest hydrocortisone (HCT) may improve survival without increasing NDD. However, the effects of HCT on head growth adjusted for illness severity during NICU hospitalization are unknown. Thus, we hypothesize that HCT will protect head growth, accounting for illness severity using a modified neonatal Sequential Organ Failure Assessment (M-nSOFA) score.
Methods
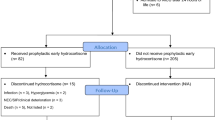
We conducted a retrospective study that included infants born at 23–29 weeks gestational age (GA) and < 1000 g. Our study included 73 infants, 41% of whom received HCT.
Results
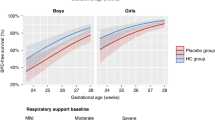
We found negative correlations between growth parameters and age, similar between HCT and control patients. HCT-exposed infants had lower GA but similar normalized birth weights; HCT-exposed infants also had higher illness severity and longer lengths of hospital stay. We found an interaction between HCT exposure and illness severity on head growth, such that infants exposed to HCT had better head growth compared to those not exposed to HCT when adjusted for illness severity.
Conclusion
These findings emphasize the importance of considering patient illness severity and suggest that HCT use may offer additional benefits not previously considered.
Impact
-
This is the first study to assess the relationship between head growth and illness severity in extremely preterm infants with extremely low birth weights during their initial NICU hospitalization.
-
Infants exposed to hydrocortisone (HCT) were overall more ill than those not exposed, yet HCT exposed infants had better preserved head growth relative to illness severity.
-
Better understanding of the effects of HCT exposure on this vulnerable population will help guide more informed decisions on the relative risks and benefits for HCT use.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Volpe, J. J. Brain injury in premature infants: A complex amalgam of destructive and developmental disturbances. Lancet Neurol. 8, 15 (2009).
Montagna, A. & Nosarti, C. Socio-emotional development following very preterm birth: Pathways to psychopathology. Front. Psychol. 7, 80 (2016).
Delobel-Ayoub, M. et al. Behavioral problems and cognitive performance at 5 years of age after very preterm birth: The EPIPAGE study. PEDIATRICS 123, 1485–1492 (2009).
Joseph, R. M. et al. Neurocognitive and Academic outcomes at age 10 years of extremely preterm newborns. Pediatrics 137, e20154343 (2016).
Thompson, D. K. et al. Tracking regional brain growth up to age 13 in children born term and very preterm. Nat. Commun. 11, 696 (2020).
Keunen, K. et al. Brain tissue volumes in preterm infants: prematurity, perinatal risk factors and neurodevelopmental outcome: A systematic review. J. Matern. Fetal Neonatal Med. 25, 89–100 (2012).
Yates, N., Gunn, A. J., Bennet, L., Dhillon, S. K. & Davidson, J. O. Preventing brain injury in the preterm infant—current controversies and potential therapies. Int. J. Mol. Sci. 22, 1671 (2021).
Ibrahim, H., Sinha, I. P. & Subhedar, N. V. Corticosteroids for treating hypotension in preterm infants. Cochrane Database Syst. Rev. 2011, CD003662 (2011).
Doyle, L. W., Ehrenkranz, R. A. & Halliday, H. L. Late (> 7 days) postnatal corticosteroids for chronic lung disease in preterm infants. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD001145.pub3. (2014).
Halliday, H. L. Update on postnatal steroids. Neonatology 111, 415–422 (2017).
Barrington, K. J. The adverse neuro-developmental effects of postnatal steroids in the preterm infant: a systematic review of RCTs. BMC Pediatr. 1, 1 (2001).
Yeh, T. F. et al. Outcomes at School Age after Postnatal Dexamethasone Therapy for Lung Disease of Prematurity. N. Engl. J. Med. 350, 1304–1313 (2004).
Parikh, N. A. et al. Postnatal dexamethasone therapy and cerebral tissue volumes in extremely low birth weight infants. Pediatrics 119, 265–272 (2007).
Cummings, J. J., Pramanik, A. K., & COMMITTEE ON FETUS AND NEWBORN. Postnatal Corticosteroids to Prevent or Treat Chronic Lung Disease Following Preterm Birth. Pediatrics e2022057530. https://doi.org/10.1542/peds.2022-057530. (2022).
Baud, O. et al. Effect of early low-dose hydrocortisone on survival without bronchopulmonary dysplasia in extremely preterm infants (PREMILOC): A double-blind, placebo-controlled, multicentre, randomised trial. Lancet 387, 1827–1836 (2016).
Onland, W. et al. Effect of hydrocortisone therapy initiated 7 to 14 days after birth on mortality or bronchopulmonary dysplasia among very preterm infants receiving mechanical ventilation: A randomized clinical trial. JAMA 321, 354 (2019).
Watterberg, K. L. et al. Hydrocortisone to improve survival without bronchopulmonary dysplasia. N. Engl. J. Med. 386, 1121–1131 (2022).
Parikh, N. A., Kennedy, K. A., Lasky, R. E., McDavid, G. E. & Tyson, J. E. Pilot randomized trial of hydrocortisone in ventilator-dependent extremely preterm infants: effects on regional brain volumes. J. Pediatr. 162, 685–690.e1 (2013).
Aziz, K. B. et al. Maximum vasoactive-inotropic score and mortality in extremely premature, extremely low birth weight infants. J. Perinatol. https://doi.org/10.1038/s41372-021-01030-9 (2021).
Lavilla, O. C. et al. Hourly kinetics of critical organ dysfunction in extremely preterm infants. Am. J. Respir. Crit. Care Med. 205, 75–87 (2022).
Cheong, J. L. Y. et al. Head growth in preterm infants: correlation with magnetic resonance imaging and neurodevelopmental outcome. PEDIATRICS 121, e1534–e1540 (2008).
Raghuram, K. et al. Head growth trajectory and neurodevelopmental outcomes in preterm neonates. Pediatrics 140, e20170216 (2017).
Fenton, T. R. & Kim, J. H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 13, 59 (2013).
Parry, G., Tucker, J. & Tarnow-Mordi, W. CRIB II: an update of the clinical risk index for babies score. Lancet 361, 1789–1791 (2003).
Aziz, K. B., Schles, E. M., Makker, K. & Wynn, J. L. Frequency of acute kidney injury and association with mortality among extremely preterm infants. JAMA Netw. Open 5, e2246327 (2022).
Fleiss, N. et al. Evaluation of the neonatal sequential organ failure assessment and mortality risk in preterm infants with late-onset infection. JAMA Netw. Open 4, e2036518 (2021).
Wynn, J. L. & Polin, R. A. A neonatal sequential organ failure assessment score predicts mortality to late-onset sepsis in preterm very low birth weight infants. Pediatr. Res. 88, 85–90 (2020).
Dorner, R. A., Burton, V. J., Allen, M. C., Robinson, S. & Soares, B. P. Preterm neuroimaging and neurodevelopmental outcome: A focus on intraventricular hemorrhage, post-hemorrhagic hydrocephalus, and associated brain injury. J. Perinatol. 38, 1431–1443 (2018).
Bakdash, J. Z. & Marusich, L. R. Repeated Measures Correlation. Front. Psychol. 8, (2017). https://doi.org/10.3389/fpsyg.2017.00456.
Papile, L. A., Burstein, J., Burstein, R. & Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1500 gm. J. Pediatr. 92, 529–534 (1978).
Morris, I. P., Goel, N. & Chakraborty, M. Efficacy and safety of systemic hydrocortisone for the prevention of bronchopulmonary dysplasia in preterm infants: A systematic review and meta-analysis. Eur. J. Pediatr. 178, 1171–1184 (2019).
Williams, E. E., Dassios, T., Mann, M. & Greenough, A. The effect of postnatal corticosteroids on growth parameters in infants with bronchopulmonary dysplasia. J. Perinat. Med. 49, 1141–1144 (2021).
Spanswick, S. C., Epp, J. R. & Sutherland, R. J. Time-course of hippocampal granule cell degeneration and changes in adult neurogenesis after adrenalectomy in rats. Neuroscience 190, 166–176 (2011).
Gould, E., Woolley, C. S. & McEwen, B. S. Short-term glucocorticoid manipulations affect neuronal morphology and survival in the adult dentate gyrus. Neuroscience 37, 367–375 (1990).
Numakawa, T. et al. Glucocorticoid receptor interaction with TrkB promotes BDNF-triggered PLC-gamma signaling for glutamate release via a glutamate transporter. Proc. Natl Acad. Sci. USA 106, 647–652 (2009).
Jeanneteau, F., Garabedian, M. J. & Chao, M. V. Activation of Trk neurotrophin receptors by glucocorticoids provides a neuroprotective effect. Proc. Natl Acad. Sci. USA 105, 4862–4867 (2008).
Bellavance, M.-A. & Rivest, S. The neuroendocrine control of the innate immune system in health and brain diseases. Immunol. Rev. 248, 36–55 (2012).
Liston, C. & Gan, W.-B. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc. Natl Acad. Sci. 108, 16074–16079 (2011).
Liston, C. et al. Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance. Nat. Neurosci. 16, 698–705 (2013).
Aucott, S. W., Donohue, P. K. & Northington, F. J. Increased morbidity in severe early intrauterine growth restriction. J. Perinatol. J. Calif. Perinat. Assoc. 24, 435–440 (2004).
Ullian, M. E. The role of corticosteroids in the regulation of vascular tone. Cardiovasc. Res. 41, 55–64 (1999).
Lösel, R. & Wehling, M. Nongenomic actions of steroid hormones. Nat. Rev. Mol. Cell Biol. 4, 46–55 (2003).
Puia-Dumitrescu, M. et al. Dexamethasone, Prednisolone, and Methylprednisolone Use and 2-Year Neurodevelopmental Outcomes in Extremely Preterm Infants. JAMA Netw. Open 5, e221947 (2022).
Acknowledgements
We thank the families of the participants and the staff at Johns Hopkins Hospital and laboratory for their willingness to participate in this study.
Funding
Supported by National Institutes of Health RO1HD086058 (A.E., F.J.N.); R01 HD110091 (F.J.N., A.D.E., K.Z., R.C-V.); RO1HD070996, AG061643, and NS109029, HD074593-07 (F.J.N.); KO8NS096115 (R.C-V.), and the Thomas Wilson Foundation (R.-V.).
Author information
Authors and Affiliations
Contributions
H.C., K.B.A., R.C-V. conceived and designed the study, analyzed the data, and drafted the article. H.C., K.B.A, H.S., S.M., A.S., S.B., A.D.E., F.J.N and R.C-V. contributed to acquisition of the data. M.G. and A.K. performed the HUS measurements. H.C., K.B.A., A.S., S.B., A.D.E., C.E.S., and R.C-V. provided critical revisions. All authors provided final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study received institutional review board approval (JHH IRB 00026068), and signed parental informed consent was obtained for each participant within the infant’s first 30 days of life and prior to NICU discharge.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, H., Aziz, K.B., Spahic, H. et al. Interaction of hydrocortisone and illness severity on head growth in cohort of ELBW infants. Pediatr Res 94, 1958–1965 (2023). https://doi.org/10.1038/s41390-023-02689-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02689-w