Abstract
Background
Acute kidney injury (AKI) is common in sick neonates and associated with poor pulmonary outcomes, however, the mechanisms responsible remain unknown. We present two novel neonatal rodent models of AKI to investigate the pulmonary effects of AKI.
Methods
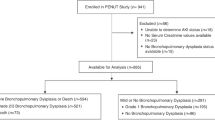
In rat pups, AKI was induced surgically via bilateral ischemia-reperfusion injury (bIRI) or pharmacologically using aristolochic acid (AA). AKI was confirmed with plasma blood urea nitrogen and creatinine measurements and kidney injury molecule-1 staining on renal immunohistochemistry. Lung morphometrics were quantified with radial alveolar count and mean linear intercept, and angiogenesis investigated by pulmonary vessel density (PVD) and vascular endothelial growth factor (VEGF) protein expression. For the surgical model, bIRI, sham, and non-surgical pups were compared. For the pharmacologic model, AA pups were compared to vehicle controls.
Results
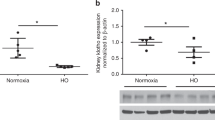
AKI occurred in bIRI and AA pups, and they demonstrated decreased alveolarization, PVD, and VEGF protein expression compared controls. Sham pups did not experience AKI, however, demonstrated decreased alveolarization, PVD, and VEGF protein expression compared to controls.
Conclusion
Pharmacologic AKI and surgery in neonatal rat pups, with or without AKI, decreased alveolarization and angiogenesis, producing a bronchopulmonary dysplasia phenotype. These models provide a framework for elucidating the relationship between AKI and adverse pulmonary outcomes.
Impact
-
There are no published neonatal rodent models investigating the pulmonary effects after neonatal acute kidney injury, despite known clinical associations.
-
We present two novel neonatal rodent models of acute kidney injury to study the impact of acute kidney injury on the developing lung.
-
We demonstrate the pulmonary effects of both ischemia-reperfusion injury and nephrotoxin-induced AKI on the developing lung, with decreased alveolarization and angiogenesis, mimicking the lung phenotype of bronchopulmonary dysplasia.
-
Neonatal rodent models of acute kidney injury provide opportunities to study mechanisms of kidney-lung crosstalk and novel therapeutics in the context of acute kidney injury in a premature infant.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Hoste, E. A. J. et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 41, 1411–1423 (2015).
Kaddourah, A., Basu, R. K., Bagshaw, S. M. & Goldstein, S. L., AWARE Investigators. Epidemiology of acute kidney injury in critically Ill children and young adults. N. Engl. J. Med. 376, 11–20 (2017).
Jetton, J. G. et al. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc. Health 1, 184–194 (2017).
Harer, M. W., Charlton, J. R., Tipple, T. E. & Reidy, K. J. Preterm birth and neonatal acute kidney injury: implications on adolescent and adult outcomes. J. Perinatol. 40, 1286–1295 (2020).
Charlton, J. R., Baldelomar, E. J., Hyatt, D. M. & Bennett, K. M. Nephron number and its determinants: a 2020 update. Pediatr. Nephrol. 36, 797–807 (2021).
White, L. E. & Hassoun, H. T. Inflammatory mechanisms of organ crosstalk during ischemic acute kidney injury. Int. J. Nephrol. 2012, 1–8 (2012).
Faubel, S. Pulmonary complications after acute kidney injury. Adv. Chronic Kidney Dis. 15, 284–296 (2008).
Basu, R. K. & Wheeler, D. S. Kidney-lung cross-talk and acute kidney injury. Pediatr. Nephrol. 28, 2239–2248 (2013).
Faubel, S. & Edelstein, C. L. Mechanisms and mediators of lung injury after acute kidney injury. Nat. Rev. Nephrol. 12, 48–60 (2016).
Alge, J. et al. Two to tango: kidney-lung interaction in acute kidney injury and acute respiratory distress syndrome. Front Pediatr. 9, 744110 (2021).
Askenazi, D. et al. Acute kidney injury is associated with bronchopulmonary dysplasia/mortality in premature infants. Pediatr. Nephrol. 30, 1511–1518 (2015).
Starr, M. C. et al. Acute kidney injury is associated with poor lung outcomes in infants born ≥ 32 weeks of gestational age. Am. J. Perinatol. 37, 231–240 (2020).
Starr, M. C. et al. Acute kidney injury and bronchopulmonary dysplasia in premature neonates born less than 32 weeks’ gestation. Am. J. Perinatol. 37, 341–348 (2020).
Northway, W. H., Rosan, R. C. & Porter, D. Y. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N. Engl. J. Med. 276, 357–368 (1967).
Alvira, C. M. & Morty, R. E. Can we understand the pathobiology of bronchopulmonary dysplasia? J. Pediatr. 190, 27–37 (2017).
Jobe, A. H. & Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 163, 1723–1729 (2001).
Kunig, A. M. et al. Recombinant human VEGF treatment transiently increases lung edema but enhances lung structure after neonatal hyperoxia. Am. J. Physiol. Lung Cell Mol. Physiol. 289, L529–L535 (2005).
Le Cras, T. D., Markham, N. E., Tuder, R. M., Voelkel, N. F. & Abman, S. H. Treatment of newborn rats with a VEGF receptor inhibitor causes pulmonary hypertension and abnormal lung structure. Am. J. Physiol. Lung Cell Mol. Physiol. 283, L555–L562 (2002).
Sharfuddin, A. A. & Molitoris, B. A. Pathophysiology of ischemic acute kidney injury. Nat. Rev. Nephrol. 7, 189–200 (2011).
Salerno, S. N. et al. Association between nephrotoxic drug combinations and acute kidney injury in the neonatal intensive care unit. J. Pediatr. 228, 213–219 (2021).
Han, J., Xian, Z., Zhang, Y., Liu, J. & Liang, A. Systematic overview of aristolochic acids: nephrotoxicity, carcinogenicity, and underlying mechanisms. Front. Pharmacol. 10, 648 (2019).
Cosyns, J. Aristolochic acid and ‘Chinese herbs nephropathy’: a review of the evidence to date. Drug Saf. 26, 33–48 (2003).
Fu, Y. et al. Rodent models of AKI-CKD transition. Am. J. Physiol. Ren. Physiol. 315, F1098–F1106 (2018).
Novitskaya, T. et al. A PTBA small molecule enhances recovery and reduces postinjury fibrosis after aristolochic acid-induced kidney injury. Am. J. Physiol. Ren. Physiol. 306, F496–F504 (2014).
Gien, J., Tseng, N., Seedorf, G., Kuhn, K. & Abman, S. H. Endothelin-1-Rho kinase interactions impair lung structure and cause pulmonary hypertension after bleomycin exposure in neonatal rat pups. Am. J. Physiol. Lung Cell Mol. Physiol. 311, L1090–L1100 (2016).
Ichimura, T., Hung, C. C., Yang, S. A., Stevens, J. L. & Bonventre, J. V. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am. J. Physiol. Ren. Physiol. 286, F552–F563 (2004).
Cooney, T. P. & Thurlbeck, W. M. The radial alveolar count method of Emery and Mithal: a reappraisal 1—postnatal lung growth. Thorax 37, 572–579 (1982).
Cooney, T. P. & Thurlbeck, W. M. The radial alveolar count method of Emery and Mithal: a reappraisal 2—intrauterine and early postnatal lung growth. Thorax 37, 580–583 (1982).
Emery, J. L. & Mithal, A. The number of alveoli in the terminal respiratory unit of man during late intrauterine life and childhood. Arch. Dis. Child. 35, 544–547 (1960).
Thurlbeck, W. M. Internal surface area and other measurements in emphysema. Thorax 22, 483–496 (1967).
Goldstein, S. L. et al. Pediatric ADQI collaborative. consensus-based recommendations on priority activities to address acute kidney injury in children: a modified delphi consensus statement. JAMA Netw. Open. 5, e2229442 (2022).
Adachi, S., Zelenin, S., Matsuo, Y. & Holtback, U. Cellular response to renal hypoxia is different in adolescent and infant rats. Pediatr. Res. 55, 485–491 (2004).
Rodriguez, M. M. et al. Histomorphometric analysis of postnatal glomerulogenesis in extremely preterm infants. Pediatr. Dev. Pathol. 7, 17–25 (2004).
Bagby, S. P. Developmental hypertension, nephrogenesis, and mother’s milk: programming the neonate. J. Am. Soc. Nephrol. 18, 1626–1629 (2007).
Schreuder, M. F., Nyengaard, J. R., Remmers, F., van Wijk, J. A. E. & Delemarre-van de Waal, H. A. Postnatal food restriction in the rat as a model for a low nephron endowment. Am. J. Physiol. 29, F1104–F1107 (2006).
Abdel-Hakeem, A. K. et al. Mechanisms of impaired nephrogenesis with fetal growth restriction: altered renal transcription and growth factor expression. Am. J. Obstet. Gynecol. 199, e1–e7 (2008).
O’Reilly, M. & Thebaud, B. Animal models of bronchopulmonary dysplasia. The term rat models. Am. J. Physiol. Lung Cell Mol. Physiol. 307, L948–L958 (2014).
Massaro, D. & Massaro, G. D. Dexamethasone accelerates postnatal alveolar wall thinning and alters wall composition. Am. J. Physiol. 251, R218–R224 (1986).
Jakkula, M. et al. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am. J. Physiol. Lung Cell Mol. Physiol. 279, L600–L607 (2000).
Sun, X. Normal and abnormal structural development of the lung. In Fetal and Neonatal Physiology (Polin, R. A., Abman, S. H., Rowitch, D. H., & Benitz, W. E. eds) 585–599 (Elsevier, Philadelphia, 2022).
Plotnikov, E. Y. et al. The role of oxidative stress in acute renal injury of newborn rats exposed to hypoxia and endotoxin. FEBS J. 284, 3069–3078 (2017).
Soni, H. & Adebiyi, A. Early septic insult in neonatal pigs increases serum and urinary soluble Fas ligand and decreases kidney function without inducing significant renal apoptosis. Ren. Fail. 39, 83–91 (2017).
Charlton, J. R. et al. Nephron loss detected by MRI following neonatal acute kidney injury in rabbits. Pediatr. Res. 87, 1185–1192 (2020).
Hoke, T. S. et al. Acute renal failure after bilateral nephrectomy is associated with cytokine-mediated pulmonary injury. J. Am. Soc. Nephrol. 18, 155–164 (2007).
Brown, C. N. et al. Surgical procedures suppress autophagic flux in kidney. Cell Death Dis. 12, 248 (2021).
Hoffman, J. et al. Sham surgery and inter-individual heterogeneity are major determinants of monocyte subset kinetics in a mouse model of myocardial infarction. PLoS One 9, e98456 (2014).
Clyman, R., Cassady, G., Kirklin, J. K., Collins, M. & Philips, J. B. The role of patent ductus arteriosus ligation in bronchopulmonary dysplasia: reexamining a randomized controlled trial. J. Pediatr. 154, 873–876 (2009).
Kabra, N. S. et al. Neurosensory impairment after surgical closure of patent ductus arteriosus in extremely low birth weight infants: results from the Trial of Indomethacin Prophylaxis in Preterms. J. Pediatr. 150, 229–234 (2007).
Garg, P. M. et al. Clinical correlates of Moderate to Severe Bronchopulmonary Dysplasia in Preterm Infants following Surgical Necrotizing Enterocolitis. Am. J. Perinatol. https://doi.org/10.1055/a-1904-9194 (2022).
Hutchens, M. P., Dunlap, J., Hurn, P. D. & Jarnberg, P. O. Renal ischemia: does sex matter? Anesth. Analg. 107, 239–249 (2008).
Soranno, D. E. et al. Female and male mice have differential longterm cardiorenal outcomes following a matched degree of ischemia-reperfusion acute kidney injury. Sci. Rep. 12, 643 (2022).
Tambunting, F., Beharry, K. D. A., Waltzman, J. & Modanlou, H. D. Impaired lung vascular endothelial growth factor in extremely premature baboons developing bronchopulmonary dysplasia/chronic lung disease. J. Investig. Med. 23, 253–262 (2005).
Maniscalco, W. M., Watkins, R. H., D’Angio, C. T. & Ryan, R. M. Hyperoxic injury decreases alveolar epithelial cell expression of vascular endothelial growth factor (VEGF) in neonatal rabbit lung. Am. J. Respir. Cell Mol. Biol. 16, 557–567 (1997).
Starr, M. C. et al. Premature infants born <28 weeks with acute kidney injury have increased bronchopulmonary dysplasia rates. Pediatr. Res. https://doi.org/10.1038/s41390-023-02514-4 (2023).
Ahuja, N. et al. Circulating IL-6 mediates lung injury via CXCL1 production after acute kidney injury in mice. Am. J. Physiol. Ren. Physiol. 303, F864–F872 (2012).
Kramer, A. A. et al. Renal ischemia/reperfusion leads to macrophage-mediated increase in pulmonary vascular permeability. Kidney Int. 55, 2362–2367 (1999).
Deng, J., Hu, X., Yuen, P. S. T. & Star, R. A. Alpha-melanocyte-stimulating hormone inhibits lung injury after renal ischemia/reperfusion. Am. J. Respir. Crit. Care Med. 169, 749–756 (2004).
Lui, K. D. et al. Predictive and pathogenetic value of plasma biomarkers for acute kidney injury in patients with acute lung injury. Crit. Care Med. 35, 2755–2761 (2007).
Lui, K. D. et al. Serum interleukin-6 and interleukin-8 are early biomarkers of acute kidney injury and predict prolonged mechanical ventilation in children undergoing cardiac surgery: a case-control study. Crit. Care. 13, R104 (2009).
Kielar, M. L. et al. Maladaptive role of IL-6 in ischemic acute renal failure. J. Am. Soc. Nephrol. 16, 3315–3325 (2005).
Klein, C. L. et al. Interleukin-6 mediates lung injury following ischemic acute kidney injury or bilateral nephrectomy. Kidney Int. 74, 901–909 (2008).
Hukriede, N. A. et al. Experimental models of acute kidney injury for translational research. Nat. Rev. Nephrol. 18, 277–293 (2022).
Arigliani, M., Spinelli, A. M., Liguoro, I. & Cogo, P. Nutrition and lung growth. Nutrients 10, 919 (2018).
Ehrenkranz, R. A. et al. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics 117, 1253–1261 (2006).
Bell, E. F. et al. Mortality, in-hospital morbidity, care practices, and 2-year outcomes for extremely preterm infants in the US, 2013-2018. JAMA 327, 248–263 (2022).
Author information
Authors and Affiliations
Contributions
B.M.L. provided substantial contributions to conception and design, acquisition of data, and analysis and interpretation of data; drafted the article; provided final approval of the version to be published. G.S. and J.G. provided substantial contributions to conception and design, acquisition of data, and analysis and interpretation of data; revised the article critically for important intellectual content; provided final approval of the version to be published. D.E.S., J.R.M., and S.G.F. provided substantial contributions to conception and design; revised the article critically for important intellectual content; provided final approval of the version to be published. A.H. provided substantial contributions to acquisition of data; revised the article critically for important intellectual content; provided final approval of the version to be published. S.H.A. revised the article critically for important intellectual content; provided final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liberio, B.M., Seedorf, G., Soranno, D.E. et al. Acute kidney injury decreases pulmonary vascular growth and alveolarization in neonatal rat pups. Pediatr Res 94, 1308–1316 (2023). https://doi.org/10.1038/s41390-023-02625-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02625-y
This article is cited by
-
Blood pressure in preterm infants with bronchopulmonary dysplasia in the first three months of life
Pediatric Nephrology (2024)
-
Advances in pediatric acute kidney injury pathobiology: a report from the 26th Acute Disease Quality Initiative (ADQI) conference
Pediatric Nephrology (2024)