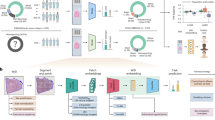
Abstract
Background
Deep learning (DL) is more and more widely used in children’s medical treatment. In this study, we have developed a computed tomography (CT)-based DL model for identifying undiagnosed non-Wilms tumors (nWTs) from pediatric renal tumors.
Methods
This study collected and analyzed the preoperative clinical data and CT images of pediatric renal tumor patients diagnosed by our center from 2008 to 2020, and established a DL model to identify nWTs noninvasively.
Results
A total of 364 children who had been confirmed by histopathology with renal tumors from our center were enrolled, including 269 Wilms tumors (WTs) and 95 nWTs. For DL model development, all cases were randomly allocated to training set (218 cases), validation set (73 cases), and test set (73 cases). In the test set, the DL model achieved area under the curve of 0.831 (95% CI: 0.712–0.951) in discriminating WTs from nWTs, with the accuracy, sensitivity, and specificity of 0.781, 0.563, and 0.842, respectively. The sensitivity of our model was higher than a radiologist with 15 years of experience.
Conclusions
We presented a DL model for identifying undiagnosed nWTs from pediatric renal tumors, with the potential to improve the image-based diagnosis.
Impact
-
Deep learning model was used for the first time to identify pediatric renal tumors in this study.
-
Deep learning model can identify non-Wilms tumors from pediatric renal tumors.
-
Deep learning model based on computed tomography images can improve tumor diagnosis rate.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to patient privacy concerns but are available from the corresponding authors on reasonable request.
References
Nakata, K. et al. Incidence of childhood renal tumours: an international population-based study. Int J. Cancer 147, 3313–3327 (2020).
Pastore, G. et al. Malignant renal tumours incidence and survival in European children (1978-1997): report from the Automated Childhood Cancer Information System project. Eur. J. Cancer 42, 2103–2114 (2006).
van den Heuvel-Eibrink, M. M. et al. Characteristics and survival of 750 children diagnosed with a renal tumor in the first seven months of life: a collaborative study by the SIOP/GPOH/SFOP, NWTSG, and UKCCSG Wilms tumor study groups. Pediatr. Blood Cancer 50, 1130–1134 (2008).
Watson, T., Oostveen, M., Rogers, H., Pritchard-Jones, K. & Olsen, Ø. The role of imaging in the initial investigation of paediatric renal tumours. Lancet Child Adolesc. Health 4, 232–241 (2020).
de la Monneraye, Y. et al. Indications and results of diagnostic biopsy in pediatric renal tumors: a retrospective analysis of 317 patients with critical review of SIOP guidelines. Pediatr. Blood Cancer 66, e27641 (2019).
Chung, E. M., Graeber, A. R. & Conran, R. M. Renal tumors of childhood: radiologic-pathologic correlation part 1. The 1st decade: from the Radiologic Pathology Archives. Radiographics 36, 499–522 (2016).
Chung, E. M. et al. Renal tumors of childhood: radiologic-pathologic correlation part 2. The 2nd decade: from the Radiologic Pathology Archives. Radiographics 37, 1538–1558 (2017).
Agrons, G. A., Kingsman, K. D., Wagner, B. J. & Sotelo-Avila, C. Rhabdoid tumor of the kidney in children: a comparative study of 21 cases. AJR Am. J. Roentgenol. 168, 447–451 (1997).
Chung, C. J. et al. Rhabdoid tumors of the kidney in children: CT findings. AJR Am. J. Roentgenol. 164, 697–700 (1995).
Lambin, P. et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 48, 441–446 (2012).
He, B. et al. Predicting response to immunotherapy in advanced non-small-cell lung cancer using tumor mutational burden radiomic biomarker. J. Immunother. Cancer 8, e000550 (2020).
Dong, D. et al. Development and validation of a novel MR imaging predictor of response to induction chemotherapy in locoregionally advanced nasopharyngeal cancer: a randomized controlled trial substudy (NCT01245959). BMC Med. 17, 190 (2019).
Dong, D. et al. Development and validation of an individualized nomogram to identify occult peritoneal metastasis in patients with advanced gastric cancer. Ann. Oncol. 30, 431–438 (2019).
Ohshima, J. et al. Methylation of the RASSF1A promoter is predictive of poor outcome among patients with Wilms tumor. Pediatr. Blood Cancer 59, 499–505 (2012).
Karmali, R. J., Suami, H., Wood, C. G. & Karam, J. A. Lymphatic drainage in renal cell carcinoma: back to the basics. BJU Int. 114, 806–817 (2014).
Pritchard-Jones, K. & Hargrave, D. Declining childhood and adolescent cancer mortality: great progress but still much to be done. Cancer 120, 2388–239 (2014).
Cattell, R. et al. Preoperative prediction of lymph node metastasis using deep learning-based features. Vis. Comput Ind. Biomed. Art. 5, 1–11 (2022).
Zhong, L. Z. et al. A deep learning MR-based radiomic nomogram may predict survival for nasopharyngeal carcinoma patients with stage T3N1M0. Radiother. Oncol. 151, 1–9 (2020).
Dong, D. et al. Deep learning radiomic nomogram can predict the number of lymph node metastasis in locally advanced gastric cancer: an international multicenter study. Ann. Oncol. 31, 912–920 (2020).
LeCun, Y., Bengio, Y. & Hinton, G. Deep learning. Nature 521, 436–444 (2015).
Zhu, X. et al. Radiomic signature as a diagnostic factor for histologic subtype classification of non-small cell lung cancer. Eur. Radio. 28, 2772–2778 (2018).
Siegel, M. J. & Chung, E. M. Wilms’ tumor and other pediatric renal masses. Magn. Reson. Imaging Clin. N. Am. 16, 479–497 (2008).
Yushkevich, P. A. et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31, 1116–1128 (2006).
Li, Q. et al. Development and validation of a deep learning algorithm to automatic detection of pituitary microadenoma from MRI. Front. Med. (Lausanne) 8, 758690 (2021).
Argani, P. et al. Clear cell sarcoma of the kidney: a review of 351 cases from the National Wilms Tumor Study Group Pathology Center. Am. J. Surg. Pathol. 24, 4–18 (2000).
Gooskens, S. L. et al. Clear cell sarcoma of the kidney: a review. Eur. J. Cancer 48, 2219–2226 (2012).
Gooskens, S. L. et al. Congenital mesoblastic nephroma 50 years after its recognition: a narrative review. Pediatr. Blood Cancer 64, 1–9 (2017).
van den Heuvel-Eibrink, M. M. et al. Malignant rhabdoid tumours of the kidney (MRTKs), registered on recent SIOP protocols from 1993 to 2005: a report of the SIOP renal tumour study group. Pediatr. Blood Cancer 56, 733–737 (2011).
Tomlinson, G. E. et al. Rhabdoid tumor of the kidney in the National Wilms’ Tumor Study: age at diagnosis as a prognostic factor. J. Clin. Oncol. 23, 7641–7645 (2005).
Aldera, A. P. & Pillay, K. Clear cell sarcoma of the kidney. Arch. Pathol. Lab. Med. 144, 119–123 (2020).
van der Beek, J. N. et al. Characteristics and outcome of pediatric renal cell carcinoma patients registered in the International Society of Pediatric Oncology (SIOP) 93-01, 2001 and UK-IMPORT database: a report of the SIOP-Renal Tumor Study Group. Int J. Cancer 148, 2724–2735 (2021).
Geller, J. I. et al. Characterization of adolescent and pediatric renal cell carcinoma: a report from the Children’s Oncology Group study AREN03B2. Cancer 121, 2457–2464 (2015).
Miniati, D. et al. Imaging accuracy and incidence of Wilms’ and non-Wilms’ renal tumors in children. J. Pediatr. Surg. 43, 1301–1307 (2008).
Shin, H. J. et al. Texture analysis to differentiate malignant renal tumors in children using gray-scale ultrasonography images. Ultrasound Med. Biol. 45, 2205–2212 (2019).
Gillies, R. J., Kinahan, P. E. & Hricak, H. Radiomics: images are more than pictures, they are data. Radiology 278, 563–577 (2016).
Napel, S., Mu, W., Jardim-Perassi, B. V., Aerts, H. & Gillies, R. J. Quantitative imaging of cancer in the postgenomic era: radio(geno)mics, deep learning, and habitats. Cancer 124, 4633–4649 (2018).
Wagner, M. W., Bilbily, A., Beheshti, M., Shammas, A. & Vali, R. Artificial intelligence and radiomics in pediatric molecular imaging. Methods 188, 37–43 (2021).
Yamashita, R., Nishio, M., Do, R. K. G. & Togashi, K. Convolutional neural networks: an overview and application in radiology. Insights Imaging 9, 611–629 (2018).
Shen, D., Wu, G. & Suk, H. I. Deep learning in medical image analysis. Annu Rev. Biomed. Eng. 19, 221–248 (2017).
Zabihollahy, F., Schieda, N., Krishna, S. & Ukwatta, E. Automated classification of solid renal masses on contrast-enhanced computed tomography images using convolutional neural network with decision fusion. Eur. Radio. 30, 5183–5190 (2020).
Han, S., Hwang, S. I. & Lee, H. J. The classification of renal cancer in 3-phase CT images using a deep learning method. J. Digit Imaging 32, 638–643 (2019).
Nguyen, K. et al. Effect of phase of enhancement on texture analysis in renal masses evaluated with non-contrast-enhanced, corticomedullary, and nephrographic phase-enhanced CT images. Eur. Radio. 31, 1676–1686 (2021).
Funding
National Natural Science Foundation of China (82022036, 91959130, 81971776, 81771924, 62027901, 81930053), the Beijing Natural Science Foundation (L182061), Strategic Priority Research Program of Chinese Academy of Sciences (XDB 38040200), the Project of High-Level Talents Team Introduction in Zhuhai City (Zhuhai HLHPTP201703), and the Youth Innovation Promotion Association CAS (2017175).
Author information
Authors and Affiliations
Contributions
Substantial contributions to conception and design: Y.Z., H.L., N.S., D.D., J.T., Y.P. Acquisition of data: Y.Z., H.L., Y.H. Analysis and interpretation of data: Y.Z., H.L., S.W. Drafting the article or revising it critically for important intellectual content: Y.Z., H.L., N.S., D.D., J.T., Y.P. Final approval of the version to be published: N.S., D.D., J.T., Y.P.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
Informed consent was obtained from all participants for the imaging examinations. Specific patient consent was not required for the publication of this paper.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, Y., Li, H., Huang, Y. et al. CT-based identification of pediatric non-Wilms tumors using convolutional neural networks at a single center. Pediatr Res 94, 1104–1110 (2023). https://doi.org/10.1038/s41390-023-02553-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02553-x