Abstract
Objective
To compare body composition and growth in very low birthweight infants according to their source of human milk: maternal expressed breast milk (MEBM) versus donor breast milk (DBM). We hypothesized that infants fed predominately MEBM would exhibit reduced body fat percentage compared to those fed predominately DBM.
Methods
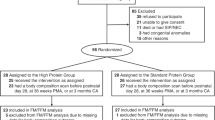
Premature infants weighing ≤1500 g on an exclusive human milk diet were enrolled in a single-center study between 2017 and 2021. Demographic data and anthropometric measurements were collected. All infants underwent body composition analysis via dual energy x-ray absorptiometry at 36 weeks corrected post menstrual age.
Results
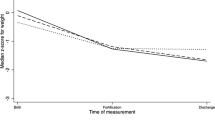
A total of 60 infants were enrolled and 48 were included in the primary analysis. No differences were detected in percent body fat (14 vs. 12%, p = 0.7) or fat-free mass (2050 vs. 2130 g, p = 0.7). Both groups displayed similar growth and anthropometric measurements. Caloric and macronutrient intake between groups was similar.
Conclusion
In the cohort of patients studied, no differences were observed in percent body fat based on primary human milk type intake in the first 28 postnatal days. Further investigation is required in a larger population of exclusive human milk fed preterm infants to determine if body composition differences exist based on an infant’s primary human milk source.
Impact
-
Premature infants are at risk for altered body composition at term corrected age, specifically increased body fat percentage, which may have implications for the future.
-
To our knowledge this is the first study exploring body composition outcomes based on an infant’s primary human milk source.
-
Infants fed exclusive human milk (e.g., donor vs. maternal) displayed similar percent body fat and growth outcomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Guellec, I. et al. Effect of intra- and extrauterine growth on long-term neurologic outcomes of very preterm infants. J. Pediatr. 175, 93–99 (2016).
Ramel, S. E. et al. Greater early gains in fat-free mass, but not fat mass, are associated with improved neurodevelopment at 1 year corrected age for prematurity in very low birth weight preterm infants. J. Pediatr. 173, 108–115 (2016).
Ramel, S. E., Haapala, J., Super, J., Boys, C. & Demerath, E. W. Nutrition, illness and body composition in very low birth weight preterm infants: implications for nutritional management and neurocognitive outcomes. Nutrients 12, 145 (2020).
Giannì, M. L. et al. Adiposity in small for gestational age preterm infants assessed at term equivalent age. Arch. Dis. Child. Fetal Neonatal Ed. 94, F368–F372 (2009).
Parlapani, E., Agakidis, C. & Karagiozoglou-Lampoudi, T. Anthropometry and body composition of preterm neonates in the light of metabolic programming. J. Am. Coll. Nutr. 37, 350–359 (2018).
Roggero, P. et al. Is term newborn body composition being achieved postnatally in preterm infants? Early Hum. Dev. 85, 349–352 (2009).
Johnson, M. J., Wooten, S. A., Leaf, A. A. & Jackson, A. A. Preterm birth and body composition at term equivalent age: a systematic review and meta-analysis. Pediatrics 130, e630–e649 (2012).
Hair, A. B. et al. Beyond necrotizing enterocolitis prevention: improving outcomes with an exclusive human milk-based diet. Breastfeed. Med. 11, 70–74 (2016).
Eidelman, A. I. et al. Breastfeeding and the use of human milk. Pediatrics 129, 827–841 (2012).
Rice, M. S. & Valentine, C. J. Neonatal body composition: measuring lean mass as a tool to guide nutrition management in the neonate. Nutr. Clin. Pract. 30, 625–632 (2015).
Lloyd, M. L., Malacova, E., Hartmann, B. & Simmer, K. A clinical audit of the growth of preterm infants fed predominantly pasteurised donor human milk v. those fed mother’s own milk in the neonatal intensive care unit. Br. J. Nutr. 121, 1018–1025 (2019).
Soldateli, B., Parker, M., Melvin, P., Gupta, M. & Belfort, M. Human milk feeding and physical growth in very low-birth-weight infants: a multicenter study. J. Perinatol. 40, 1246–1252 (2020).
Montjaux-Régis, N. et al. Improved growth of preterm infants receiving mother’s own raw milk compared with pasteurized donor milk. Acta Paediatr. 100, 1548–1554 (2011).
Cerasani, J. et al. Human milk feeding and preterm infants’ growth and body composition: a literature review. Nutrients 12, 1155 (2020).
Visuthranukul, C., Abrams, S. A., Hawthorne, K. M., Hagan, J. L. & Hair, A. B. Premature small for gestational age infants fed an exclusive human milk-based diet achieve catch-up growth without metabolic consequences at 2 years of age. Arch. Dis. Child. Fetal Neonatal Ed. 104, F242–F247 (2019).
American College of Obstetricians and Gynecologists' Committee on Practice Bulletins—Obstetrics and the Society forMaternal-FetalMedicin. ACOG Practice Bulletin No. 204: Fetal Growth Restriction. Obstet. Gynecol. 133, e97–e109 (2019).
Janisse, J. J., Bailey, B. A., Ager, J. & Sokol, R. J. Alcohol, tobacco, cocaine and marijuana use: relative contributions to preterm delivery and fetal growth restriction. Subst. Abus. 35, 60–67 (2014).
van Wyk, L. et al. Postnatal catch-up growth after suspected fetal growth restriction at term. Front. Endocrinol. 10, 274 (2019).
Prolacta Bioscience®. Preterm nutrition products. Prolact HM® Product Specification Sheet. https://www.prolacta.com/en/products/preterm-nutrition-products/ (2018).
Hologic. Horizon DXA System. Body composition. https://www.hologic.com/hologic-products/body-composition/horizon-dxa-system#230548828-3749065048 (2022).
Brunton, J. A., Bayley, H. S. & Atkinson, S. A. Validation and application of dual-energy x-ray absorptiometry to measure bone mass and body composition in small infants. Am. J. Clin. Nutr. 58, 839–845 (1993).
Prolacta Bioscience®. Preterm nutrition products. Nutrition Information 100% Human milk-based neonatal nutritional products from prolacta bioscience. https://www.prolacta.com/en/products/preterm-nutrition-products/ (2021).
Fenton, T. R. et al. Accuracy of preterm infant weight gain velocity calculations vary depending on method used and infant age at time of measurement. Pediatr. Res. 85, 650–654 (2019).
Ezz-Eldin, Z. M., Hamid, T. A. A., Youssef, M. R. L. & Nabil, H. E. Clinical Risk Index for Babies (CRIB II) Scoring System in prediction of mortality in premature babies. J. Clin. Diagnostic Res. 9, 8–11 (2015).
Fenton, T. R. & Kim, J. H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 13, 1–13 (2013).
Zhang, Z. Missing data imputation: focusing on single imputation. Ann. Transl. Med. 4, 1–9 (2016).
Mcnelis, K. et al. Body composition of very low-birth-weight infants fed fortified human milk: a pilot study. J. Parenter. Enter. Nutr. 45, 784–791 (2021).
Perrin, M. T. et al. The nutritional composition and energy content of donor human milk: a systematic review. Adv. Nutr. 11, 960–970 (2021).
de Halleux, V. & Rigo, J. Variability in human milk composition: benefit of individualized fortification in very-low-birth-weight infants. Am. J. Clin. Nutr. 98, 529S–535S (2013).
Donath, S. M. & Amir, L. H. Does maternal obesity adversely affect breastfeeding initiation and duration? J. Paediatr. Child Health 36, 482–486 (2000).
Torloni, M. R. et al. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obes. Rev. 10, 194–203 (2009).
Chu, S. Y. et al. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care 30, 2070–2076 (2007).
Borràs-Novell, C. et al. Infrared analyzers for the measurement of breastmilk macronutrient content in the clinical setting. Expert Rev. Mol. Diagnostics 20, 867–887 (2020).
Acknowledgements
We thank University Health Systems in San Antonio, Texas and the University Hospital Neonatal Nutrition and Bone Institute for their support and guidance throughout this study. We thank the University Hospital NICU respiratory therapists who supervised the subjects during the DXA scans to ensure patient safety. In addition, we thank the parents of the neonates enrolled and the patients who selflessly contributed of themselves to our scientific pursuit and knowledge acquisition.
Funding
This study was funded by Dr Cynthia Blanco’s Greehey Family Foundation Chair in Neonatology Research and University Health System Foundation Grant. A.G.M. is supported by Eunice Kennedy Shriver National Institute Of Child Health & Human Development of the National Institutes of Health under Award Number K23HD101701. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
C.B.R. was responsible for designing the study, obtaining IRB approval, screening and enrolling subjects, collecting data, interpreting results, and writing the manuscript. K.L.M. was responsible for completing IRB progress reports and amendments, screening and enrolling subjects, collecting data, interpreting results, and writing the manuscript. R.J., E.L., and K.B. contributed to screening and enrolling subjects, collecting data, and provided feedback on the manuscript. D.A.G. contributed to data extraction and analysis and provided feedback on the manuscript. S.K. contributed to data entry. J.G. assisted with statistical analysis. D.M. and C.L.B. contributed to the study design and provided feedback on the manuscript. A.G.M. is the primary investigator and provided feedback and assistance with the study design, statistical analysis, and manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
C.L.B. has received financial support from Prolacta Bioscience® for an unrelated research project. The products described in this study were part of standard of care at University Health Systems. Prolacta Bioscience® provided no financial support or scientific input for this study. The remaining authors of this study have no conflicts of interest to disclose.
Consent to participate
Parental consent was obtained prior to enrollment, or any research activities were performed.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ramirez, C.B., McCoy, K.L., Jacob, R. et al. Effects of human milk on body composition and growth in very low birthweight infants. Pediatr Res 93, 2028–2035 (2023). https://doi.org/10.1038/s41390-022-02364-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02364-6