Abstract
Background
Late and moderate preterm (LMPT) infants are at risk for adverse later life outcomes. We determined the association between feeding method at enrolment and growth and body composition of LMPT infants until 3 months corrected age (3mCA).
Methods
Infants born between 32+0 and 36+6 weeks of gestation (n = 107) were enrolled up to 4 weeks corrected age and stratified according to feeding at enrolment. We performed anthropometric measurements at enrolment, term equivalent age (TEA) and 3mCA, including skinfold measurements and body composition using dual X-ray absorptiometry (DEXA).
Results
Feeding method at enrolment was associated with fat mass (FM) (breast 554.9 g, mixed 716.8 g, formula 637.7 g, p = 0.048), lean body mass (LM) (2512 g, 2853 g, 2722 g, respectively, p = 0.009) and lean mass index (LMI) (10.6 kg/m2, 11.6 kg/m2,11.2 kg/m2 respectively, p = 0.008) at TEA, but not 3mCA. Breastfed infants demonstrated greater increase in LM (breast 1707 g, mixed 1536 g, formula 1384 g, p = 0.03) and LMI (1.23 kg/m2, 0.10 kg/m2, 0.52 kg/m2, respectively, p = 0.022) between TEA and 3mCA.
Conclusions
Breastfed LMPT infants have lower FM and greater LM increase and LMI increase up to 3mCA compared to formula or mixed-fed infants. These findings stress the importance of supporting breastfeeding in this population.
Impact
-
Infants born late and moderate preterm age who are exclusively breastfed soon after birth gain more lean mass up to 3 months corrected age compared to mixed- or formula-fed infants.
-
Breastfed infants have lower lean and fat mass at term equivalent age compared to mixed- and formula-fed infants.
-
This is the first study exploring this population’s growth and body composition in detail at 3 months corrected age.
-
Our results underline the importance of supporting mothers to initiate and continue breastfeeding at least until 3 months corrected age.
Similar content being viewed by others
Introduction
Moderate preterm (MPT, 32–33 weeks of gestation) and late preterm (LPT) babies (34–36 weeks of gestation) represent 84.7% of all preterm births.1 Compared to those born at term, MPT and LPT infants have increased health risks in early and later life, including worse neurodevelopmental and metabolic health outcomes.2,3,4
Several studies have indicated that the long-term trajectory of cognitive and metabolic development is associated with growth and body composition development in early life, highlighting the importance of this as a sensitive period for later life health risks associated with preterm birth.5,6,7,8 Faster length and body mass index (BMI) gain in infancy in preterm infants is also associated with reduced risk for lower intelligence quotient (IQ) in childhood and early adulthood,7 whereas poor growth is associated with adverse neurodevelopmental outcomes.9 On the other hand, rapid weight gain in infancy is associated with adverse metabolic outcomes.5,6,9,10,11,12 This underlines the importance of balanced growth in infancy that may optimise neurodevelopment without jeopardising adverse metabolic outcomes. The limited data available on growth patterns in late and moderate preterm (LMPT, 32+0–36+6 weeks of gestation) suggest that they differ from term infants throughout the first years of life.13,14,15,16,17,18 Longitudinal data are required to determine the potential health impact of these observations.
Adequate nutrition plays a key role in optimal growth and development in all preterm infants. Breastfeeding is the preferred source of nutrition for preterm infants, including LMPT infants,19 as it has beneficial impacts on body composition and cognitive outcomes.20,21 However, data relating to LMPT infants is scarce and greater understanding of the impact of early life nutrition on growth and body composition of LMPT infants is needed.
The FLAMINGO study (Feeding Late and Moderate Preterm Infants and Growth Outcomes) is a longitudinal observational study on growth, body composition and feeding patterns of LMPT infants in the first 2 years of life. The primary aim of this study was to study growth and body composition from term equivalent age (TEA) until 3 months corrected age (3mCA) in a cohort of LMPT infants in relation to the feeding method at the time of enrolment and gestational age.
Subjects and methods
The population characteristics and methods of the FLAMINGO study have been described in detail previously.22
Study population
Infants born in the Royal Victoria Infirmary, Newcastle upon Tyne, UK were screened for potential enrolment in the study. Eligible infants were those born between 32+0 and 36+6 weeks of gestation, with birth weight 1.25–3.0 kg, who lived within travelling distance from the research facility and were less than 4 weeks corrected age (44 weeks post-menstrual age). Infants were enrolled regardless of their location, the Neonatal Unit, postnatal ward or occasionally after discharge from hospital. Infants with medical problems likely to affect growth (such as genetic disorders, congenital anomalies, heart defects, etc.) or significant social problems were excluded. As per study protocol, introduction of solid foods was withheld until at least 3mCA.
The size of the overall study population to be recruited was determined by the numbers needing to be enrolled to a randomized controlled trial (RCT) nested within the overall cohort.22 This nested non-inferiority RCT comparing weight gain between 2 formula-fed groups assumed a daily weight gain of 3 g/day as adequate, was powered at 80% and would have required 140 infants assuming an attrition rate of 30%. In addition to these 140 infants, we estimated we would recruit an additional 110 infants who were breastfed and would not therefore be eligible for the nested RCT. The study presented here represents those recruited to the overall cohort during the time available.
Data collected
At the time of initial parental consent (visit 1), we collected baseline anthropometric and demographic characteristics, including the residential postcode that is used to calculate an index of multiple deprivation (IMD).23 Infants had further appointments at TEA (visit 2) and at 3mCA (visit 3). If enrolment was after TEA, then the starting visit was visit 2. During those visits, we performed detailed anthropometric measurements, including skinfolds, collected data and questionnaires on their feeding method and assessed body composition using dual X-ray absorptiometry (DEXA).
Anthropometric measurements were conducted by two investigators and included the infants’ weight, length, head circumference, mid-upper arm circumference (left side), mid-thigh circumference (left side) and skinfolds (triceps, biceps, subscapular and supra-iliac), and we calculated BMI and growth z-scores. The World Health Organization (WHO) standards do not provide z-scores for mid-upper arm circumference, triceps and subscapular skinfolds at TEA and there are no available z-scores for mid-thigh circumference, biceps and supra-iliac skinfolds for TEA or 3 months.24 The mid-upper arm circumference and the triceps skinfolds were used to calculate the arm muscle area, arm fat area and arm fat percentage (Supplementary Fig. 1). DEXA was used to estimate the lean body mass (LM), total body fat mass (FM), lean mass percentage (LM%), fat mass percentage (FM%), lean mass index (LMI), fat mass index (FMI), bone mineral density (BMD) and bone mineral content (BMC). Changes over time and z-scores changes between TEA and 3mCA were calculated along with fractional weight gain (g/kg/day), head circumference gain (cm/week) and length (cm/week) gain velocities for this period (Supplementary Fig. 1).
Infants were included in the analyses only if weight was available for both TEA and 3mCA. If these infants did not have other anthropometric measurements, then they were not included in the relevant analysis.
Statistical analysis
Analyses were conducted to explore relationships between type of feeding at enrolment and outcomes hypothesised to be associated with feeding type.
Infants were allocated to a feeding group based on the type of milk reported by their mothers at enrolment: breastfed (receiving up to one supplementary formula feed a day), formula fed (receiving no more than one breastfeed a day) or mixed fed. We compared the feeding groups to evaluate the impact of the feeding type on growth and body composition changes between TEA and 3mCA.
The z-scores for anthropometric measurements were based on the 1990 WHO Growth data on the UK prior to TEA25 and the most recent WHO data on term infants for values close to and after TEA.24 The use of two databases does not affect the presented results, as the outcome data collected on the key timepoints of interest, TEA and 3mCA, were based on the same (latter) database.
Changes between TEA and 3mCA in growth and body composition were compared between the groups. An analysis of variance was used to test for statistically significant differences among all three feeding groups for continuous variables with a normal distribution, t tests to compare continuous variables with a normal distribution between two groups. Normality was based on Shapiro–Wilk test. Kruskal–Wallis tests or Wilcoxon–Mann–Whitney U-tests, as appropriate, were used to compare non-normally distributed continuous variables and chi-square tests for categorical variables. We explored the effect of outliers on the findings and did not find that these were affecting the results. A p value of <0.05 was considered statistically significant.
Further stratified analyses were performed to explore the impact of potentially influential factors on growth outcomes, including the gestational age at birth (stratified to MPT, born between 32+0 and 33+6, and LPT, born between 34+0 and 36+6) and the maternal socioeconomic background, using the IMD (Supplementary Material).23
The statistical package used for analyses of data was Stata 16.0.26 The STROBE cohort reporting guidelines were used.27
Results
Study population
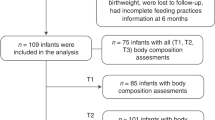
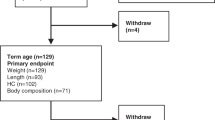
The initial target was to enrol 250 infants in the cohort, based on the power calculation described in the protocol.22 In March 2020 the enrolment stopped, secondary to the coronavirus disease 2019 (COVID-19) pandemic and end of funding. Between May 2018 and March 2020, a total of 182 LMPT infants were recruited (Fig. 1), of which 107 infants (59%) completed both the TEA and 3mCA visits and were included in the analyses. The median age (interquartile range (IQR)) at the time of enrolment was 36+3 weeks gestational age (IQR 2 weeks). Ninety-three of these infants were enrolled prior to TEA. Their birth and demographic characteristics are presented in Table 1 and show approximately equal numbers of boys and girls, with approximately half of the infants born via caesarean section. The mean gestational age at birth was 35+2 weeks (24 infants were MPT and 83 infants were LPT) and the mean birth weight was 2.24 kg. The mean maternal age was 33 years (standard deviation [SD] 5.41), median maternal BMI before pregnancy was 24.6 kg/m2 (25th centile: 22.0, 75th centile:30.1) and 60 mothers (56.0%) lived in the fourth and fifth most socio-economically deprived areas according to IMD quintiles (Supplementary Table 1).
The cohort of 107 infants did not differ significantly with the full population of infants born in the above period for birth weight (p = 0.791), GA (p = 0.489) or sex (p = 0.552). However, there was significant difference for the IMD (p = 0.022 for chi-square test). Our cohort had more infants in the most deprived quintile compared to the full population (17.8% compared to 10.7%, respectively) and less infants from the least deprived group (25.2% compared to 39.9%, respectively).
Anthropometrics, skinfold measurements and body composition measurements of the study population at TEA and 3mCA are shown in Table 2. Only 14 out of 107 infants were enrolled after TEA and their measurements at enrolment were used as their TEA measurements and standardised for their corrected age with z-scores. At TEA and 3mCA, most growth parameters had negative mean z-scores, except for head circumference for age. Between TEA and 3mCA, there were no significant changes in weight-for-age, BMI-for-age, head circumference-for-age and length-for-age z-scores (0.05, 0.25, 0.17 and 0.10, respectively). There were no significant effects on catch-up or catch-down growth (change in centile position). From TEA to 3mCA, all skinfold thickness area measurements increased significantly. However, at 3mCA, mean mid–upper arm circumference, triceps and subscapular skinfolds z-scores were still below the mean (−0.19 [SD 1.12], −1.13 [SD 1.16] and −0.99 [SD 0.96], respectively).
Between TEA and 3mCA, the DEXA scans (Table 3) showed that both total mean LM and mean FM increased (by 1584 g and 1158 g, respectively), with the mean FM% increasing (from 18.7% at TEA to 29.3% at 3mCA) matched as expected by a parallel decrease in mean LM% (change from 81.3% to 70.7%). The mean FMI also increased (2.63 kg/m2 [SD 0.98] at TEA to 4.94 kg/m2 [SD 0.89] at 3mCA), whereas there was only a marginal increase in the LMI (11.1 kg/m2 [SD1.34] at TEA and 11.8 kg/m2 [SD 0.99] at 3 months). Finally, both mean BMD (0.29 g/cm2 [SD 0.03] to 0.33 g/cm2 [SD 0.04]) and mean BMC (55.8 g [SD 11.4] to 103.9 g [SD 15.8]) increased from TEA to 3mCA.
Impact of milk feeding type
The groups stratified by the mode of feeding were similar in size (breastfed n = 41, formula fed n = 33, mixed fed n = 33). Baseline characteristics of the three groups were similar except for a higher number of days of hospital stay for the breastfeeding compared to the formula feeding group (10.7 [SD 8.3] and 6.8 [SD 5.6], respectively, p = 0.024, Table 4).
Body composition assessed by DEXA showed that the feeding groups had significantly different FM (p = 0.034), LM (p = 0.009) and LMI (p = 0.008) at TEA with the breastfed group having lower values (Table 5). Furthermore, at TEA the breastfeeding group had lower values for triceps (p = 0.024), biceps (p = 0.074) and supra-iliac (p = 0.071) skinfolds and arm fat% (p = 0.004) compared to the mixed- and formula-fed groups and also when compared directly to the formula-fed group (p values 0.045, 0.029. 0.028 and 0.01, respectively) (Table 5). At 3mCA, these differences were no longer significant when using either DEXA or skinfold measurements (Table 5), in line with the apparent greater increase in mean LM and mean LMI in the period between TEA to 3mCA in the breastfed group compared to the other two feeding groups (Table 6).
At 3mCA, the breastfed group had significantly greater mean weight compared to the formula-fed group (5.81 kg [SD 1.01] and 5.64 kg [SD 0.61], respectively, p = 0.005), but similar to the mixed-fed group (5.92 kg [SD 0.76], p = 0.11). At TEA, there was no significant difference.
Between TEA and 3mCA, the only significant differences in anthropometric changes among the 3 groups were the mean increase of LM (breastfed 1707 g, mixed fed 1536 g, formula fed 1384 g, p = 0.003) and the mean increase of LMI (breastfed 1.23 kg/m2, mixed fed 0.10 kg/m2, formula fed 0.52 kg/m2, p = 0.022). A head-to-head comparison between the breastfed to the formula-fed group showed a significant difference in mean change in weight-for-age z-score (0.24 and −0.24, respectively, p = 0.025), LM (1707 g and 1384 g, respectively, p = 0.005) and LMI (1.23 kg/m2 and 0.52 kg/m2, respectively, p = 0.049) between TEA and 3mCA (Table 6).
Since the LM and FM at TEA were significantly different, we explored their role as a cofounder in the body composition changes. Therefore, we performed further analyses, adjusting the body composition changes between TEA and 3mCA, for the LM and FM at TEA (Table 7). As the LM and FM are highly correlated (0.8), we performed two separate analyses. The FM change became significantly higher in the breastfed compared to the formula-fed group following these adjustments. The changes in LM remained significantly different only when comparing breastfed to formula-fed groups, adjusting for LM at TEA. The changes in LMI were no longer significant.
Impact of gestational age and socio-economic status
There was a strong negative correlation between the gestational age and the length of hospital stay (−0.773; p < 0.001). The analyses and stratification as per gestational age group (MPT versus LPT) or maternal socio-economic status showed small changes in mean z-scores between TEA and 3mCA in all groups, with no statistically significant differences for these values between the compared stratified groups (Supplementary Tables 2 and 3).
Discussion
In a cohort of 107 LMPT infants, we have shown substantial changes in body composition from TEA to 3mCA, whereas weight and length growth was stable, as suggested by marginal changes of z-scores over time. In addition, our findings support that the type of milk feeding at the time of enrolment is an important factor influencing growth, body composition and their velocities in the first months of life, with breastfed infants having lowest FM and LM at TEA and the most rapid LM gain up to 3mCA.
In line with previous findings in a cohort of 122 LPT infants,13 we observed negative z-scores at TEA for all anthropometric outcomes, with the exception of the head circumference-for-age. In contrast, Roggero et al. found that LPT infants had greater weight at TEA compared to term infants,28 although the mean weight of the term infants in their cohort was still below the mean of the WHO standards. The lower than median z-score values observed in these two studies28,29 and our study may be a consequence of preterm birth itself or might be explained by the fact that, unlike our study, the WHO standards are based on exclusively breastfed infants.
In the current study, we found a significant impact of the type of milk at enrolment feeding on body composition at TEA, with the breastfed group demonstrating the lowest mean LM, LMI and FM and the mixed-fed group the highest. However, these differences seem to have been transient and at 3mCA no longer statistically significant. Gianni et al. showed a positive association of (any) breastfeeding with fat-free mass content in LPT at TEA,30 which is in contrast with our findings that breastfed infants had the lowest FM and LM at TEA, although their relative values were similar to formula-fed infants. However, it fits with the most rapid LM gain up to 3mCA in the breastfed infants suggesting a positive effect of breastfeeding on body composition till 3mCA.
Our results on growth velocity between TEA to 3mCA suggest an adequate growth pattern in this study population, since none of the infants showed either rapid catch-up or faltering growth. Actually, the mean values of each of the z-scores in the cohort only showed small changes between the two time points (Fig. 2 and Table 2). This agrees with Zhao et al. who found that MPT and LPT compared to term infants have increased velocity for weight and length until 3mCA but no catch-up growth.31 Additionally, stratification based on type of milk feeding showed that there was no significant difference in growth velocities among the feeding groups of the current study, apart from a higher weight-for-age z-score gain for the breastfed infants compared to the formula-fed group. The more detailed analysis of body composition changes between TEA and 3mCA showed that the breastfed group had the largest gain in LM and LMI among the three feeding groups, as well as when compared directly with the formula-fed group. At TEA, the breastfed infants had the lowest LM and FM and they only demonstrated accelerated gain for LM, whereas the gain in FM was not different to the other feeding groups. They also had significantly greater gain in LM when compared solely to formula fed infants, which is responsible for the observed difference in weight gain between the two groups (according to weight-for-age change). This suggests that exclusive breastfeeding in early life provides an advantage for LMPT infants in the quality of body composition changes until 3mCA. However, breastfeeding initiation and continuation rates in LMPT infants are lower compared to either extremely preterm or term infants32,33,34,35,36 because of the barriers to breastfeeding that this population faces.34,36,37,38,39 Moreover, balanced nutritional support of LMPT infants remains largely unexplored, resulting in marked variation in clinical practices including supplementation and milk type, and the lack of evidence-based guidelines.40,41,42
Previous work in preterm infants has underlined the importance of early life growth and body composition in later neurodevelopmental and metabolic outcomes.5,6,7,10,11,43 This is especially important for this population who, compared to term infants, have significant risks for higher FM and lower LM.44 Pfister et al. and Ramel et al. found that faster LM gain is associated with improved neurodevelopmental outcomes of preterm infants in childhood.5,45 Furthermore, Scheurer et al. found an association between the velocity of increase of LM in the first 4 months of age of preterm infants with preschool LM with potential links to later life beneficial metabolic outcomes.46 In our study, the breastfed infants had larger gain in LM compared to the other feeding groups. Our findings suggest that changes in body composition of exclusively breastfed LMPT infants, may result in favourable metabolic and developmental outcomes compared to infants with different feeding patterns, in line with the results of Johnson et al.47 Moreover, our results provide potential explanations for the work by Yan et al. who found that breastfeeding had a protective role against obesity.20 In summary, our study showed differences in body composition at TEA in LMPT infants receiving different milk feeding patterns, as well as in the amount of LM gain until 3mCA, with the breastfed infants gaining weight faster and more LM, which may be linked to beneficial later life outcomes.
Our study has several strengths. To our knowledge, this is the first study to explore the impact of the type of milk feeding on growth and body composition of LPT and MPT, beyond TEA. Gianni et al. also explored the impact of breast milk, but only in LPT and only at TEA.30 Our methods of data collection were extensive and conducted to high standards with all measurements conducted by two investigators, using the same equipment and with robust follow-up at specific time points. We used DEXA to assess directly body composition and we measured skinfolds, which have not been used previously to provide a comprehensive assessment of growth and body composition in LMPT infants. We have used z-scores where available to compare the groups’ characteristics, as this is a more reliable comparison method. This was not possible for the body composition measurements (lean and fat mass), as there are no standardised values for these.
However, our study also has certain limitations. Our study suffered from important attrition losses that was in part due to the COVID-19 pandemic (41.2% at 3mCA, Fig. 1). Furthermore, the majority of participants were self-defined as from one ethnic group (White British, defined per the National Health Service). However, we did achieve significant representation from relatively deprived socio-economic groups. The feeding groups were dynamic and overlapping, as mothers changed the type of milk between the enrolment and the third visit. However, we stratified infants strictly according to the type of milk at the time of enrolment as this accurately reflects the reality in the general population. Finally, due to the size of the cohort we were not able to perform multiple regression to account for confounding; alternatively we have performed stratified analyses to explore separately the effect of different factors on growth and body composition.
In conclusion, we provide detailed information on the postnatal growth of an understudied population and the influence of the type of milk feeding in the first weeks of life on growth and body composition development. We have shown that infants breastfed at enrolment, compared to other types of feeding, have lower LM and FM at TEA, which is weeks after their birth. They also had significantly greater gain in LM until 3mCA. When adjusting for LM and FM at TEA, the gain of FM was also significantly bigger in the breastfed group compared to the formula-fed group. Given the importance of early infancy growth on later-life outcomes, our findings suggest that breastfeeding promotes greater infant LM gain, which has been shown to be associated with favourable neurodevelopmental outcomes and well-balanced body composition development. Our study highlights the importance of adequately supporting mothers of LMPT infants to initiate and continue breastfeeding in this vulnerable population.
Data availability
The data sets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Chawanpaiboon, S. et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob. Health 7, e37–e46 (2019).
Karnati, S., Kollikonda, S. & Abu-Shaweesh, J. Late preterm infants - changing trends and continuing challenges. Int. J. Pediatr. Adolesc. Med. 7, 36–44 (2020).
Johnson, S. et al. Differentiating the preterm phenotype: distinct profiles of cognitive and behavioral development following late and moderately preterm birth. J. Pediatr. 193, 85.e1–92.e1 (2018).
Boyle, E. M. et al. Effects of gestational age at birth on health outcomes at 3 and 5 years of age: population based cohort study. BMJ 344, e896 (2012).
Pfister, K. M. et al. Early body composition changes are associated with neurodevelopmental and metabolic outcomes at 4 years of age in very preterm infants. Pediatr. Res. 84, 713–718 (2018).
Belfort, M. B., Gillman, M. W., Buka, S. L., Casey, P. H. & McCormick, M. C. Preterm infant linear growth and adiposity gain: trade-offs for later weight status and intelligence quotient. J. Pediatrics 163, 1564–1569.e2 (2013).
Sammallahti, S. et al. Growth after late-preterm birth and adult cognitive, academic, and mental health outcomes. Pediatr. Res. 81, 767–774 (2017).
Cordova, E. G. et al. Association of poor postnatal growth with neurodevelopmental impairment in infancy and childhood: comparing the fetus and the healthy preterm infant references. J. Pediatr. 225, 37–43.e5 (2020).
Euser, A. M. et al. Associations between prenatal and infancy weight gain and BMI, fat mass, and fat distribution in young adulthood: a prospective cohort study in males and females born very preterm. Am. J. Clin. Nutr. 81, 480–487 (2005).
Reilly, J. J. et al. Early life risk factors for obesity in childhood: cohort study. BMJ 330, 1357 (2005).
de Fluiter, K. S., van Beijsterveldt, I., Breij, L. M., Acton, D. & Hokken-Koelega, A. Association between fat mass in early life and later fat mass trajectories. JAMA Pediatr. 174, 1141–1148 (2020).
Druet, C. et al. Prediction of childhood obesity by infancy weight gain: an individual-level meta-analysis. Paediatr. Perinat. Epidemiol. 26, 19–26 (2012).
Giannì, M. L. et al. Postnatal catch-up fat after late preterm birth. Pediatr. Res. 72, 637–640 (2012).
Gupta, P. et al. Physical growth, morbidity profile and mortality among healthy late preterm neonates. Indian Pediatr. 54, 629–634 (2017).
Santos, I. S. et al. Late preterm birth is a risk factor for growth faltering in early childhood: a cohort study. BMC Pediatr. 9, 71 (2009).
Dotinga, B. M. et al. Longitudinal growth and emotional and behavioral problems at age 7 in moderate and late preterms. PLoS ONE 14, e0211427 (2019).
Bocca-Tjeertes, I. F., Kerstjens, J. M., Reijneveld, S. A., de Winter, A. F. & Bos, A. F. Growth and predictors of growth restraint in moderately preterm children aged 0 to 4 years. Pediatrics 128, e1187–e1194 (2011).
Goyal, N. K., Fiks, A. G. & Lorch, S. A. Persistence of underweight status among late preterm infants. Arch. Pediatr. Adolesc. Med. 166, 424–430 (2012).
Lapillonne, A. et al. Feeding the late and moderately preterm infant: a Position Paper of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 69, 259–270 (2019).
Yan, J., Liu, L., Zhu, Y., Huang, G. & Wang, P. P. The association between breastfeeding and childhood obesity: a meta-analysis. BMC Public Health 14, 1267 (2014).
Anderson, J. W., Johnstone, B. M. & Remley, D. T. Breast-feeding and cognitive development: a meta-analysis. Am. J. Clin. Nutr. 70, 525–535 (1999).
Kakaroukas, A. et al. An observational cohort study and nested randomized controlled trial on nutrition and growth outcomes in moderate and late preterm infants (FLAMINGO). Front. Nutr. 8, 561419 (2021).
Ministry of Housing, Communities & Local Government. English indices of deprivation 2019. https://www.gov.uk/government/statistics/english-indices-of-deprivation-2019 (2019).
WHO. WHO Anthro for personal computers [Internet]. Child growth standards. Geneva; 2010. Available from: http://www.who.int/childgrowth/software/en/
Pan, H. & Cole T. LMSgrowth. http://www.healthforallchildren.co.uk/ (2012).
StataCorp. Stata Statistical Software (StataCorp LLC., 2022).
von Elm E. et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. BMJ 335, 806 (2007).
Roggero, P., Giannì, M. L., Liotto, N., Piemontese, P. & Mosca, F. Late preterm infants’ growth and body composition after discharge. Ital. J. Pediatr. 40, A27 (2014).
Giannì, M. L. et al. Postnatal catch-up fat after late preterm birth. Pediatr. Res. 72, 637–640 (2012).
Giannì, M. L. et al. Does human milk modulate body composition in late preterm infants at term-corrected age? Nutrients 8, 664 (2016).
Zhao, Z. et al. Trajectories of length, weight, and bone mineral density among preterm infants during the first 12 months of corrected age in China. BMC Pediatr. 15, 91 (2015).
Maastrup, R. et al. Breastfeeding progression in preterm infants is influenced by factors in infants, mothers and clinical practice: the results of a national cohort study with high breastfeeding initiation rates. PLoS ONE 9, e108208 (2014).
Demirci, J. R., Sereika, S. M. & Bogen, D. Prevalence and predictors of early breastfeeding among late preterm mother-infant dyads. Breastfeed. Med. 8, 277–285 (2013).
Goyal, N. K., Attanasio, L. B. & Kozhimannil, K. B. Hospital care and early breastfeeding outcomes among late preterm, early-term, and term infants. Birth 41, 330–338 (2014).
Jónsdóttir, R. B., Jónsdóttir, H., Skúladóttir, A., Thorkelsson, T. & Flacking, R. Breastfeeding progression in late preterm infants from birth to one month. Matern. Child Nutr. 16, e12893 (2020).
Nagulesapillai, T., McDonald, S. W., Fenton, T. R., Mercader, H. F. & Tough, S. C. Breastfeeding difficulties and exclusivity among late preterm and term infants: results from the all our babies study. Can. J. Public Health 104, e351–e356 (2013).
Giannì, M. L. et al. Facilitators and barriers of breastfeeding late preterm infants according to mothers’ experiences. BMC Pediatr. 16, 179 (2016).
Carpay, N. C., Kakaroukas, A., D Embleton, N. & van Elburg, R. M. Barriers and facilitators to breastfeeding in moderate and late preterm infants: a systematic review. Breastfeed. Med. 16, 370–384 (2021).
Kair, L. R. & Colaizy, T. T. Breastfeeding continuation among late preterm infants: barriers, facilitators, and any association with NICU admission? Hospital Pediatr. 6, 261–268 (2016).
Blackwell, M. T. et al. Interneonatal intensive care unit variation in growth rates and feeding practices in healthy moderately premature infants. J. Perinatol. 25, 478–485 (2005).
Mitha, A. et al. The impact of neonatal unit policies on breast milk feeding at discharge of moderate preterm infants: the EPIPAGE-2 cohort study. Matern. Child Nutr. 15, e12875 (2019).
McCormick, M. C., Escobar, G. J., Zheng, Z. & Richardson, D. K. Place of birth and variations in management of late preterm (“near-term”) infants. Semin. Perinatol. 30, 44–47 (2006).
Stettler, N., Kumanyika, S. K., Katz, S. H., Zemel, B. S. & Stallings, V. A. Rapid weight gain during infancy and obesity in young adulthood in a cohort of African Americans. Am. J. Clin. Nutr. 77, 1374–1378 (2003).
Hamatschek, C. et al. Fat and fat-free mass of preterm and term infants from birth to six months: a review of current evidence. Nutrients 12, 288 (2020).
Ramel, S. E. et al. Greater early gains in fat-free mass, but not fat mass, are associated with improved neurodevelopment at 1 year corrected age for prematurity in very low birth weight preterm infants. J. Pediatr. 173, 108–115 (2016).
Scheurer, J. M. et al. Body composition trajectories from infancy to preschool in children born premature versus full-term. J. Pediatr. Gastroenterol. Nutr. 64, e147–e153 (2017).
Johnson, S. et al. Neurodevelopmental outcomes following late and moderate prematurity: a population-based cohort study. Arch. Dis. Child. Fetal Neonatal Ed. 100, F301–F308 (2015).
Acknowledgements
We are very thankful to the Research Nursing staff of the Royal Victoria Infirmary for assisting with the enrolment and data collection for the study. The data analysis was performed by independent statisticians who have no conflict of interest to declare.
Funding
This research was funded by Danone Nutricia Research via an Investigator Led Clinical Research Funding award to Newcastle Hospitals NHS Foundation Trust.
Author information
Authors and Affiliations
Contributions
M.A.-B., N.D.E. and R.M.v.E. concepted and designed the study. A.K., J.E.B. and N.D.E. acquired data. L.H. and R.J.Q.M.N. performed the statistical analysis and interpretation was done by all authors. A.K., M.A.-B., N.D.E., R.M.v.E. and J.E.B. drafted the article. All authors took part revising the paper. A.K., M.A.-B., N.D.E. and R.M.v.E. had primary responsibility for the final content. All authors read and approved the final version of manuscript.
Corresponding author
Ethics declarations
Competing interests
M.A.-B. is an employee of Danone Nutricia Research. R.M.v.E. was an employee of Danone Nutricia Research during conduct of this study. N.D.E. and J.E.B. declare research funding from Danone Early Life Nutrition and Prolacta Biosciences US and lecture honoraria from Nestle Nutrition Institute. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethics approval and consent to participate
The study was reviewed by the North East–York Research Ethics Committee, approved by the NHS Health Research Authority (IRAS project ID: 237542) and registered at ISRCTN (ISRCTN15469594). The procedures followed were in accordance with the Helsinki Declaration of 1975 as revised in 1983. Consent was sought and obtained according to the standards of ethical research. Parents of eligible infants were approached from the time of birth up to 4 weeks after term equivalent age and given a study leaflet, explained the study and answered questions and approached again at a second stage to answer questions and explore intention for consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kakaroukas, A., Abrahamse-Berkeveld, M., Hayes, L. et al. Early infancy growth, body composition and type of feeding in late and moderate preterms. Pediatr Res 93, 1927–1935 (2023). https://doi.org/10.1038/s41390-022-02317-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02317-z