Abstract
Background
Successful pregnancies are nowadays possible after kidney transplantation but are associated with a higher incidence of maternal and fetal complications. Immunosuppressive therapy causes cardiovascular side effects but must be maintained during pregnancy. Little is known about the consequences of maternal kidney transplantation on offspring’s endothelial health. Endothelial colony forming cells (ECFCs) represent a highly proliferative subtype of endothelial progenitor cells and are crucial for vascular homeostasis, repair and neovascularization. Therefore, we investigated whether maternal kidney transplantation affects fetal ECFCs’ characteristics.
Methods
ECFCs were isolated from umbilical cord blood of uncomplicated and post-kidney-transplant pregnancies and analyzed for their functional abilities with proliferation, cell migration, centrosome orientation and angiogenesis assays. Further, ECFCs from uncomplicated pregnancies were exposed to either umbilical cord serum from uncomplicated or post-kidney-transplant pregnancies.
Results
Post-kidney-transplant ECFCs showed significantly less proliferation, less migration and less angiogenesis compared to control ECFCs. The presence of post-kidney-transplant umbilical cord serum led to similar functional aberrations of ECFCs from uncomplicated pregnancies.
Conclusions
These pilot data demonstrate differences in ECFCs’ biological characteristics in offspring of women after kidney transplantation. Further studies are needed to monitor offspring’s long-term cardiovascular development and to assess possible causal relationships with immunosuppressants, uremia and maternal cardiovascular alterations.
Impact
-
Pregnancy after kidney transplantation has become more common in the past years but is associated with higher complications for mother and offspring.
-
Little is known of the impact of maternal kidney transplantation and the mandatory immunosuppressive therapy on offspring vascular development.
-
In this study we are the first to address and detect an impairment of endothelial progenitor cell function in offspring of kidney-transplanted mothers.
-
Serum from post-transplant pregnancies also causes negative effects on ECFCs’ function.
-
Clinical studies should focus on long-term monitoring of offspring’s cardiovascular health.
Similar content being viewed by others
Introduction
Since the first pregnancy resulted in a live birth following a kidney transplantation with consecutive immunosuppression in 1967, the number of female transplant recipients of child-bearing age has steadily increased and the issue of post-transplantation pregnancies has become considerably more important1. Due to modern immunosuppressive drugs, the risk of rejection has decreased and the fertility of women often restores after kidney transplantation2. Nevertheless, the management of these pregnancies is challenging and associated with higher rates of maternal and perinatal complications3.
Life-long immunosuppression is necessary to avoid graft rejection but might present a potential hazard for the offspring during pregnancy and afterwards. Most immunosuppressive drugs reach the fetal circulation by crossing the placental barrier4.
It is well-known that the intrauterine milieu and complications during pregnancy co-determine offspring’s health and cardiovascular risk. While exposure to adverse conditions, e.g. preeclampsia and diabetes, is associated with cardiovascular impairment in children in later life, data on offspring exposed to immunosuppressants in utero are limited and the impact of maternal transplantation and immunosuppressive therapy during pregnancy on offspring’s cardiovascular health has not been studied yet5,6,7,8,9,10,11,12,13,14.
Endothelial progenitor cells (EPCs) are impaired in several cardiovascular diseases15,16,17 and are considered as one of the strongest biomarkers to evaluate endothelial dysfunction and cardiovascular risk18,19,20,21. Endothelial colony-forming cells (ECFCs), a highly proliferative subgroup of EPCs, play an important role in angiogenesis and vascular repair and contribute to endothelial integrity22,23.
At the time of birth, cord blood-derived ECFCs are easily accessible and functional impairment has been reported in pregnancy complications which are associated with long-term cardiovascular impairment of the offspring24,25,26. In this study, we therefore tested whether fetal ECFCs are affected in pregnancies following maternal kidney transplantation.
Materials and methods
Patients
The study was approved by the Institutional Review Board of Hannover Medical School (approval no. 1443–2012 and 504–2009). Written informed consent was obtained from each participant.
Umbilical cord blood was collected from 12 uncomplicated and 6 post-kidney-transplant pregnancies for ECFC isolation, serum extraction or both. Uncomplicated pregnancies were defined as normotensive and without proteinuria, preexisting diabetes, hypertensive, vascular or renal disease, smoking or the use of illicit drugs ending with the delivery of a healthy baby. For the experiments, patients were matched by maternal age, BMI and gestational age at delivery.
ECFC isolation, culture and characterization
Umbilical cord blood was collected into sterile EDTA-tubes immediately after delivery. Serum was extracted from separate serum tubes and stored at −80 °C until use for further experiments. ECFCs were isolated as previously described25,27,28 and cultured in endothelial growth medium 2 (EGM-2) consisting of endothelial basal medium (EBM-2; Lonza, Basel, Switzerland) supplemented with supplier provided supplements, 10% fetal bovine serum (FBS; Harvard Bioscience, Holliston, MA) and 1% penicillin/streptomycin (P/S; Bio&Sell, Feucht, Nürnberg, Germany) at 37 °C, 5% CO2. Day of appearance of ECFC colonies and total colony number were evaluated. ECFC colonies were noted as circumscribed cell monolayers with cobblestone-like morphology. Well-defined colonies were expanded and characterized by flow cytometry with typical phenotype markers (CD31 + , CD45-, and CD133-) by using appropriate antibodies (CD31, 130-117-390, BD Biosciences, San Jose, CA; CD45, 555483, BD Biosciences; and CD133, 130-090-826, Miltenyi Biotec, Bergisch Gladbach, Germany) and corresponding isotype controls (BD Biosciences, Miltenyi Biotec). ECFCs were used for experiments in cell culture passages 4–6.
Cell impedance assay
For continuous monitoring of live cell proliferation, morphology and viability we used the xCelligence system (Roche, Basel, Switzerland), an impedance-based real-time analysis. The change in impedance is measured via the cell index, a dimensionless parameter reflecting cell adhesion, migration and proliferation and was calculated with the xCelligence Real-Time Cell Analyzer. The electrical impedance caused by adherent cells is converted into cell indices by the xCelligence software (v.1.2.1)28.
For ECFC comparison, 0.25 × 104 cells from 4 control and 4 post-transplant ECFC lines were seeded in quadruplicates in EGM-2 with 10% FBS and 1% P/S onto a gold-coated E-Plate View 96-well plate (Roche) and then placed into the Real-Time Cell Analyzer SP station, positioned in a 37 °C incubator with 5% CO2 supply. Following adherence, the cell indices were aligned for all lines and then continuously monitored for 72 h.
To analyze serum effects, 0.25 × 104 cells from 5 control ECFC lines were seeded in quadruplicates in EGM-2 with 2.5% FBS and 1% P/S and analyzed as described above. When a stable cell index was reached, 2.5% pooled control or pooled transplant serum were added to the cells. Cell impedance was recorded for 72 h.
In vitro angiogenesis assay
To compare the capacity to form capillary tubule-like networks, 1.4 × 104 cells/well from 4 control and 4 post-transplant ECFC lines were incubated in triplicates in 96-well plates pre-coated with 30 µL growth factor reduced Matrigel (BD Biosciences) for 14 h in EBM-2 with 5% FBS and 1% P/S24.
In separate experiments, 5 control ECFC lines were treated with either 5% control or 5% transplant serum in EBM-2 with 1% P/S. Phase contrast microscopic images were taken with a Leica DMI 6000 B microscope (Leica, Wetzlar, Germany). Total tube length and number of branch points in each visual field were calculated with ImageJ 1.50b (National Institutes of Health)26. Branch points were defined as nodes with connections to at least 3 tubes.
Migration assay
To assess migration ability 5 × 104 cells from 4 control and 4 post-transplant ECFC lines were seeded on gelatin-coated (Sigma-Aldrich, St. Louis, Missouri) wells of 6-well culture plates with EGM-2 containing 10% FBS and 1% P/S and grown to confluence. The cell monolayers were scratched with a sterile pipette tip to create a wound and washed with PBS. Afterwards, cells were cultured in fresh EBM-2 with 2.5% FBS and 1% P/S.
To analyze serum effects on ECFCs’ migration ability, 5 control ECFC lines were seeded, grown and scratched as described above. Then 5% control or transplant serum was added to the medium. Phase contrast microscopic images were immediately taken after scratching and then again after 18 h with a Leica DMI 6000 B microscope. Non-populated scratch areas were quantified by ImageJ 1.50b and subtracted to obtain the remigrated area.
Centrosome orientation assay
In several cell types, there is a correlation between the position of the centrosome and the direction of cell movement: the centrosome is located behind the leading edge, suggesting that it serves as a control device for directional cell movement29. A change in the direction of cell movement precedes a re-orientation of the centrosome in the intended direction and is a sign of polarity of migrating cells30. During migration, the centrosome is positioned between the nucleus and the leading part, indicating the migrational status and direction31. To study differences in centrosome orientation, ECFCs were grown to confluence on coverslip glasses in 6-well culture plates and scratches were performed. After 1 h, cells were washed with PBS, fixed with 3% formaldehyde and 2% sucrose and permeabilized with 0.2% Triton X-100 (Sigma-Aldrich). Cells were incubated with an antibody against pericentrin (ab28144; Abcam, Cambridge, UK) in 2% normal goat serum (Thermo Fisher Scientific, Waltham, Massachusetts) and PBS for 2 h, washed 3x with PBS and incubated with Alexa Fluor anti-mouse IgG 546 (Thermo Fisher Scientific) for 2 h. Nuclear DNA was stained with 4′,6-diamidino-2-phenylindole (DAPI; Thermo Fisher Scientific), and coverslip glasses were mounted in mounting medium (ProLongGold; Thermo Fisher Scientific). Images were acquired randomly along the scratch with a Leica DMI 6000 B microscope (Leica).
Statistical analysis
Normality distribution was tested by Shapiro-Wilk or D’Agostino normality test. Students paired t test was applied for serum associated data, students unpaired t test was used to analyze cell related data. Welch’s correction was administered in case of unequal variances. Not normally distributed data were assessed by Wilcoxon matched-pairs signed rank test or Mann Whitney test, respectively. Experimental data of biological replicates are presented as mean and standard error in the text. The obtained individual measured values (n) from each experiment were analyzed with Prism 9 (GraphPad Software, La Jolla, CA). P-values at < 0.05 were considered statistically significant and indicated in the figures as follows: * p < 0.05, ** p < 0.01.
Results
Patient characteristics
The kidney transplantation related clinical data for the transplanted women recruited for the study are given in Supplemental Table 1. The average time that has elapsed since transplantation was 5.5 years ± 1.5 years. All transplanted women received immunosuppression with tacrolimus. The mean tacrolimus plasma concentration was 5.4 µg/l ± 0.4 µg/l. All concentrations were in line with the average concentrations reported by Hebert et al.32
The comparison of pregnancy associated clinical and demographic data for women who provided umbilical cord blood are given in Table 1. Maternal age, gravidity, parity, maternal pre-pregnancy BMI, birth weight, birth weight percentile, delivery mode and sex of the baby were not statistically different between the control and the transplant group. The transplanted women had higher blood pressures at delivery (systolic: 144.7 ± 9.6 mmHg; diastolic: 87.3 ± 5.1 mmHg) compared to the control group (systolic: 112.9 ± 2.4 mmHg, p < 0.001; diastolic: 68.0 ± 1.6 mmHg, p = 0.004), although none had developed preeclampsia. The mean maternal serum creatinine concentration of the transplant group (Tx, 124 ± 11 µmol/l) was significantly higher than the control group (Con, 53 ± 3 µmol/l, p = 0.001) whereas the mean GFR was significantly lower (Tx 55 ± 9 ml/min/1,73 m2 vs. Con 123 ± 3 ml/min/1,73 m2, p < 0.001), respectively.
Lower cell index of fetal ECFCs under transplant conditions
The time to the appearance of the first ECFC colony (Tx 8.5 ± 0.9 days vs. Con 9.2 ± 2.4 days, n = 4–5, p = 0.81) and the total number of colonies formed (Tx 9.0 ± 3.2 colonies vs. Con 13.4 ± 5.4 colonies, n = 4–5, p = 0.53) did not differ between the control and the transplant group.
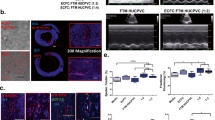
The cell index of offspring from women with a kidney transplant, determined by real-time cell analysis, kept up with ECFCs derived from control umbilical cord blood in the first 24 h (Tx 22,149 ± 2,596 vs. Con 23,196 ± 1216, n = 4, p = 0.73), slowed down after 48 h (Tx 33,610 ± 8,250 vs. Con 46,468 ± 2,118, n = 4, p = 0.18) and showed a significantly lower increase after 72 h (Tx 35,240 ± 10,006 vs. 61,979 ± 4,164, n = 4, p = 0.049), Fig. 1a.
a Overlay of growth curves of ECFCs from healthy or post-transplant pregnancies. The cell index of transplant ECFCs is significantly lower after 72 h. b Overlay of growth curves of ECFCs treated with control or transplant serum. The presence of transplant serum leads to a significantly lower cell index after 24, 48 and 72 h. n = 4–5; Con control, Tx transplant, CS control serum, TS transplant serum. * p < 0.05, ** p < 0.01.
In the presence of transplant serum (TS) the cell index was markedly lower when compared to the incubation with serum from healthy controls (CS) (TS 16,209 ± 1,592 vs. CS 24,681 ± 1,871 after 24 h, p = 0.005; TS 27,470 ± 4,239 vs. CS 43,005 ± 5,986 after 48 h, p = 0.008; TS 38,962 ± 7,623 vs. CS 55,689 ± 10,260 after 72 h, p = 0.04, n = 5), Fig. 1b.
Reduced tube formation ability of fetal ECFCs after maternal transplantation and after incubation with transplant cord serum
An in vitro angiogenesis assay was performed to reflect ECFCs’ ability to form de novo vessels in vivo. ECFCs derived from umbilical cord blood from transplant patients showed significantly lower tube lengths and lower number of branch points than ECFCs derived from umbilical cord blood from control patients (tube lengths: Tx 3.02 × 107 µm ± 0.70 × 107 µm vs. Con 4.93 × 107 µm ± 0.34 × 107 µm, n = 4, p = 0.048; branch points: Tx 41 ± 13 vs. Con 106 ± 17, n = 4, p = 0.02), Fig. 2a, b.
a ECFCs of post-transplant pregnancies show less tube formation ability. b Representative images of control (a) and transplant (b) ECFCs. c Incubation with transplant serum impairs ECFCs’ angiogenesis. d Representative images of ECFCs treated with control (c) or transplant serum (d). Images were obtained after 14 h. Scale bar 500 µm. Box plots represent median, 25th and 75th percentile, whiskers the minimum and the maximum. n = 4–5. Con control, Tx transplant, CS control serum, TS transplant serum. * p < 0.05.
ECFCs’ ability to form capillary-like structures in Matrigel was significantly impaired in presence of umbilical cord serum from transplant patients in comparison to umbilical cord serum from healthy controls (TS 3.22 × 107 µm ± 0.42 × 107 µm vs. CS 3.62 × 107 µm ± 0.44 × 107 µm, n = 5, p = 0.047). The difference in the number of branch points did not reach significance (Tx: 52 ± 10 vs. Con 62 ± 11, n = 5, p = 0.30), Fig. 2c, d.
Impaired migration of fetal ECFCs after maternal transplantation and after incubation with transplant cord serum
We addressed the migration capacity of transplant patients’ offspring’s ECFCs in a scratch wound healing assay. Transplant ECFCs showed significantly less wound closure after 18 h than control ECFCs (relative remigrated area: Tx 0.72 ± 0.08 vs. Con 1.00 ± 0.12, n = 4, p = 0.045), Fig. 3a, b.
a ECFCs from post-transplant pregnancies are less capable to migrate. b Representative images of control (a) and transplant (b) ECFCs after 18 h of migration. c Incubation with transplant serum impairs ECFC migration. d Representative images of ECFCs treated with control (c) or transplant serum (d). Scale bar 1000 µm. Box plots represent median, 25th and 75th percentile, whiskers the minimum and the maximum. Cell-free area after 18 h was subtracted from cell-free area at start to calculate remigrated area. Mean of control group was set to 1. n = 4–5. Con control, Tx transplant, CS control serum, TS transplant serum. * p < 0.05, ** p < 0.01.
In presence of transplant serum, ECFCs remigrated about half as much of the area as when incubated with control serum (relative remigrated area: TS 0.46 ± 0.07 vs. CS 1.00 ± 0.07, n = 5, p = 0.001), Fig. 3c, d.
Delayed pro-migratory positioning of fetal ECFCs from kidney transplanted women
Pericentrin staining was applied to gain insight in centrosome orientation. In accordance to the findings in the scratch wound healing assay, the proportion of ECFCs in direction towards the wound as well as the ratio of forwards and backwards directed cells were significantly lower in the transplant group compared to the control group (forwards: Tx 0.48 ± 0.02, n = 4 vs. Con 0.68 ± 0.04, n = 7, p = 0.006; backwards: Tx 0.52 ± 0.02, n = 4, vs. Con 0.32 ± 0.04, n = 7, p = 0.006; ratio: Tx 0.94 ± 0.06, n = 4, vs. Con 2.38 ± 0.34, n = 7, p = 0.01), Fig. 4.
Centrosome localization is indicated by pericentrin staining (red) in immunofluorescence 1 h after the scratch performance. Nuclei were counterstained with DAPI (blue). Transplant ECFCs show less forwards (a) and more backwards (b) orientation than control ECFCs. Ratio of forwards and backwards orientation is calculated in c. Representative images of control and transplant ECFCs are shown in d and e. White lines indicate scratch borders. White arrows show the migration direction of the cells: forwards (towards the scratch) and backwards (away from the scratch). At least 37 cells were counted per line. Scale bar 50 µm. Box plots represent median, 25th and 75th percentile, whiskers the minimum and the maximum. n = 4–7, Con control, Tx transplant. * p < 0.05, ** p < 0.01.
Discussion
In this study, we report a significant impairment of main biological characteristics of fetal ECFCs from mothers with a kidney transplant. A similar effect was observed when ECFCs derived from healthy pregnancies were exposed to umbilical cord serum from pregnancies after maternal kidney transplantation. To our knowledge, we are the first to address endothelial progenitor cells in transplant patients’ offspring as surrogate marker for vascular health.
The foundation for cardiovascular diseases in later life can already be laid during pregnancy7,9. Therefore, it is pivotal to identify risk factors for cardiovascular health as early as possible, when classical risk factors are not yet visible. This might pave the way for early intervention and primary prevention. Pregnancies after kidney transplantation carry a higher risk for the development of pregnancy complications, e.g. pregnancy-induced hypertension, preeclampsia, IUGR, and preterm birth33—well-known risk factors for future cardiovascular impairment of mothers and offspring12,34,35,36.
Fetal EPCs, which are considered to be involved in vascular homeostasis and repair37, appear to be an adequate model in this context to study vascular health. Our findings are in line with previous studies which describe an impairment of offspring ECFC number and function in gestational diseases or in the newborn period which are associated with later cardiovascular impairment of the progeny. In infants with bronchopulmonary dysplasia, a lung disease associated with prematurity, decreased numbers of ECFCs were reported38. In preeclampsia, a hypertensive disorder of pregnancy, the number of ECFC colonies was lower compared to controls39 and cells showed reduced proliferation, migrated less40 and formed fewer tubules24 which corresponds to our findings in transplant ECFCs. Recently, another study drew the link between ECFCs, neonatal complications and future cardiovascular diseases. It was demonstrated that in former preterms elevated systolic blood pressure significantly correlated with alterations in ECFC proliferation and tube formation41. These findings support our assumption that ECFCs are a suitable marker for vascular impairment.
We additionally demonstrated a negative effect on ECFCs derived from healthy pregnancies when incubated with cord serum of transplant pregnancies compared to cord serum of healthy controls. It seems possible that this observation may be the consequence of a substance circulating in the materno-placental-fetal system. The underlying disease that led to the transplantation is often associated with numerous cardiovascular risk factors and end organ damage, which only partially regress after the transplantation. The post-kidney-transplant cohort in our study still showed significantly higher concentrations of creatinine as an example for circulating urinary substances. In this context, EPCs have shown to be reduced in numbers, function and differentiation in chronic kidney disease patients as well as in uremia42,43. Another potential cause for our observations is the mandatory use of immunosuppressive agents, e.g. tacrolimus. Adverse effects include hypertension, hypercholesterinemia and hyperglycemia with the corresponding effects on the vascular system, leading to endothelial dysfunction44. Rabbits exposed to calcineurin inhibitors in utero were reported to be asymptomatic at birth, but presented hypertension, proteinuria and chronic kidney disease in adulthood implying possible long-term effects of intrauterine exposure to calcineurin inhibitors14,45. Regarding pregnancies in humans, it has been reported that, while trying to maintain target whole blood concentrations, dosage titration leads to a considerable increase of unbound tacrolimus concentrations, which is suggested to have important clinical implications46. Tacrolimus can accumulate in placenta as well as in ex vivo perfused placental tissue what could be associated with cytotoxic effects on placental level47. Unfortunately, the amount of serum obtained from umbilical cord was insufficient to record the concentration of tacrolimus or other metabolites in the newborns included in our study. However, we recently demonstrated calcineurin inhibitor induced functional impairment of ECFCs already in therapeutic concentrations28. These findings support our hypothesis of a possible contribution of immunosuppressive medication to reduced ECFC function in offspring of transplant patients. Apart from circulating substances themselves, there might also be a role for exosomes derived from endothelial or circulating cells. It has been shown that conditioned media or exosomes derived from ECFCs of patients with a known cardiovascular disease led to dysregulation of migration and impairment of tube formation of healthy ECFCs. It was stated that this effect is possibly mediated via the introduction of RNAs including miRNAs48.
In general, the pathogenesis of fetal complications is difficult to assess as there are many interacting factors such as the intrauterine exposure to immunosuppressive agents, higher incidence of preterm birth as well as the concomitant maternal pathologies like high blood pressure that can influence the fetal outcome14,24,49. Although not significant, mean gestational age was shorter in the transplant recipients in our study. This could have influenced the study results, but the literature is not clear on this. Baker et al. reported that preterm cord blood grew more ECFC colonies due to a higher proliferative capacity than term blood did and ECFCs had a similar angiogenic capacity50. This is in line with higher numbers of ECFC colonies in the study by Munoz-Hernandes et al.39 but in contrast to the findings by Ligi et al. who describe similar ECFC colony numbers but impaired function of preterm ECFCs51. It is worth noting that three out of six women in the transplant group have taken low-dose acetylsalicylic acid (ASA), which in pregnancy is used in the prevention of preeclampsia. In this context Hu et al. found a favorably impact on EPC migratory function and on the prevention of senescence using low-dose ASA52. At high-dose ASA they and Chen et al. observed an impairment of EPC function52,53. Considering the positive effect of low-dose ASA in clinical but also in in vitro studies on vascular and endothelial health one would expect this to be reflected in ECFC characteristics of the transplant cohort. However, the effects of the aberrant milieu in transplant recipients seem to mask the favorable impact of ASA in our study. Altogether, the history of kidney transplantation and the complications mentioned cannot be discussed independently of one another and should be understood as invitation for interdisciplinary thinking.
Although the number of pregnancies after kidney transplantation has increased, the single-center experience still remains quite low, leading to a small sample size in our pilot study. As in the latter we already detected considerable effects, we wish to share our results to encourage other researchers to contribute to the further elucidation of underlying mechanisms. Confirmatory studies including more participants are needed to corroborate our results and to better reflect the kidney transplant patients’ variety. Also, higher sample sizes would allow to adjust the results for the impact of potential confounders, e.g. gestational age at delivery. The transplant population of our study displayed heterogenous characteristics. For the six women included there were five different causes that led to the former kidney failure. Further, the time between transplantation and pregnancy differed from 2 to 10 years. The validity of our results is correspondingly limited. Another limitation of our study is reflected in the short assay time. Our in vitro analyses covered a period from 14 to 72 h, so no conclusions can be drawn about long-term effects.
So far, there are very few long-term follow-up studies targeting children of transplanted mothers. Most of the available information is limited essentially to classical parameters such as height, weight and head circumference monitoring which fortunately were unremarkable in the majority of children14,54,55. Nevertheless, as systemic alterations after intrauterine calcineurin inhibitor exposure were detected only in adult rodents45, Boulay et al. consequently concluded that the lack of symptoms in children might not be predictive of the absence of long-term effects14. Therefore, and considering the results of our study, further efforts are clearly needed to get a broader picture of possible consequences of maternal transplantation on their offspring’s cardiovascular health. Continuous multidisciplinary long-term follow-up studies should be implemented to open up the possibility for early interventions when facing cardiovascular risk.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Armenti, V. T., Constantinescu, S., Moritz, M. J. & Davison, J. M. Pregnancy after transplantation. Transplant. Rev. 22, 223–240 (2008).
Davison, J. M. Dialysis, transplantation, and pregnancy. Am. J. Kidney Dis. 17, 127–132 (1991).
Le, H. L. et al. Usage of tacrolimus and mycophenolic acid during conception, pregnancy, and lactation, and its implications for therapeutic drug monitoring: a systematic critical review. Therapeutic drug Monit. 42, 518–531 (2020).
Venkataramanan, R. et al. Cyclosporine and its metabolites in mother and baby. Transplantation 46, 468–469 (1988).
Gluckman, P. D., Hanson, M. A., Cooper, C. & Thornburg, K. L. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 359, 61–73 (2008).
Barker, D. J. Maternal and fetal origins of coronary heart disease. J. R. Coll. Physicians Lond. 28, 544–551 (1994).
Barker, D. J. In utero programming of cardiovascular disease. Theriogenology 53, 555–574 (2000).
Barker, D. J. Fetal programming of coronary heart disease. Trends Endocrinol. Metab. 13, 364–368 (2002).
Ehr, Jvon & Versen-Höynck, Fvon Implications of maternal conditions and pregnancy course on offspring’s medical problems in adult life. Arch. Gynecol. Obstet. 294, 673–679 (2016).
Nahum Sacks, K. et al. Prenatal exposure to preeclampsia as an independent risk factor for long-term cardiovascular morbidity of the offspring. Pregnancy hypertension 13, 181–186 (2018).
Øglaend, B., Forman, M. R., Romundstad, P. R., Nilsen, S. T. & Vatten, L. J. Blood pressure in early adolescence in the offspring of preeclamptic and normotensive pregnancies. J. Hypertension 27, 2051–2054 (2009).
Davis, E. F. et al. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics 129, e1552–61 (2012).
Jayet, P.-Y. et al. Pulmonary and systemic vascular dysfunction in young offspring of mothers with preeclampsia. Circulation 122, 488–494 (2010).
Boulay, H. et al. Maternal, foetal and child consequences of immunosuppressive drugs during pregnancy in women with organ transplant: a review. Clin. Kidney J. 14, 1871–1878 (2021).
Michowitz, Y. et al. Circulating endothelial progenitor cells and clinical outcome in patients with congestive heart failure. Heart 93, 1046–1050 (2007).
Andreou, I., Tousoulis, D., Tentolouris, C., Antoniades, C. & Stefanadis, C. Potential role of endothelial progenitor cells in the pathophysiology of heart failure: clinical implications and perspectives. Atherosclerosis 189, 247–254 (2006).
Luo, S., Xia, W., Chen, C., Robinson, E. A. & Tao, J. Endothelial progenitor cells and hypertension: current concepts and future implications. Clin. Sci. 130, 2029–2042 (2016).
Hill, J. M. et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N. Engl. J. Med. 348, 593–600 (2003).
Sen, S., McDonald, S. P., Coates, P. T. H. & Bonder, C. S. Endothelial progenitor cells. Novel biomarker and promising cell therapy for cardiovascular disease. Clin. Sci. 120, 263–283 (2011).
Fadini, G. P., Losordo, D. & Dimmeler, S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circulation Res. 110, 624–637 (2012).
Vasa, M. et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circulation Res. 89, E1–7 (2001).
Ingram, D. A. et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 104, 2752–2760 (2004).
Collett, J. A. et al. Endothelial colony-forming cells ameliorate endothelial dysfunction via secreted factors following ischemia-reperfusion injury. Am. J. Physiol. Ren. Physiol. 312, F897–F907 (2017).
Versen-Höynck, F., von, Brodowski, L., Dechend, R., Myerski, A. C. & Hubel, C. A. Vitamin D antagonizes negative effects of preeclampsia on fetal endothelial colony forming cell number and function. PloS ONE 9, e98990 (2014).
Gui, J. et al. Vitamin D rescues dysfunction of fetal endothelial colony forming cells from individuals with gestational diabetes. Placenta 36, 410–418 (2015).
Brodowski, L. et al. Vitamin D prevents endothelial progenitor cell dysfunction induced by sera from women with preeclampsia or conditioned media from hypoxic placenta. PloS ONE 9, e98527 (2014).
Grundmann, M. et al. Vitamin D improves the angiogenic properties of endothelial progenitor cells. Am. J. Physiol. Cell Physiol. 303, C954–62 (2012).
Meyer, N., Brodowski, L., Kaisenberg, Cvon, Schröder-Heurich, B. & Versen-Höynck, Fvon Cyclosporine A and tacrolimus induce functional impairment and inflammatory reactions in endothelial progenitor cells. IJMS 22, 9696 (2021).
Ueda, M., Gräf, R., MacWilliams, H. K., Schliwa, M. & Euteneuer, U. Centrosome positioning and directionality of cell movements. Proc. Natl Acad. Sci. USA 94, 9674–9678 (1997).
Luxton, G. W. G. & Gundersen, G. G. Orientation and function of the nuclear-centrosomal axis during cell migration. Curr. Opin. cell Biol. 23, 579–588 (2011).
Schliwa, M., Euteneuer, U., Gräf, R. & Ueda, M. Centrosomes, microtubules and cell migration. Biochemical Soc. Symp. 65, 223–231 (1999).
Hebert, M. F. et al. Interpreting tacrolimus concentrations during pregnancy and postpartum. Transplantation 95, 908–915 (2013).
Shah, S. et al. Pregnancy outcomes in women with kidney transplant: Metaanalysis and systematic review. BMC Nephrol. 20, 24 (2019).
Levent, E. et al. The relation of arterial stiffness with intrauterine growth retardation. Pediatrics Int.: Off. J. Jpn. Pediatr. Soc. 51, 807–811 (2009).
Szostak-Węgierek, D., Szamotulska, K. & Maj, A. Relationship between carotid intima-media thickness, atherosclerosis risk factors and birthweight in young males. Kardiologia Pol. 69, 673–678 (2011).
Craici, I., Wagner, S. & Garovic, V. D. Preeclampsia and future cardiovascular risk: formal risk factor or failed stress test? Therapeutic Adv. cardiovascular Dis. 2, 249–259 (2008).
Medina, R. J. et al. Endothelial progenitors. A consensus statement on nomenclature. Stem cells Transl. Med. 6, 1316–1320 (2017).
Baker, C. D. et al. Cord blood angiogenic progenitor cells are decreased in bronchopulmonary dysplasia. Eur. respiratory J. 40, 1516–1522 (2012).
Muñoz-Hernandez, R. et al. Decreased level of cord blood circulating endothelial colony-forming cells in preeclampsia. Hypertension 64, 165–171 (2014).
Gumina, D. L., Black, C. P., Balasubramaniam, V., Winn, V. D. & Baker, C. D. Umbilical cord blood circulating progenitor cells and endothelial colony-forming cells are decreased in preeclampsia. Reprod. Sci. 24, 1088–1096 (2017).
Bertagnolli, M. et al. Endothelial colony-forming cells in young adults born preterm. a novel link between Neonatal complications and adult risks for cardiovascular disease. J. Am. Heart Assoc. 7; https://doi.org/10.1161/JAHA.118.009720 (2018).
Bahlmann, F. H., Speer, T. & Fliser, D. Endothelial progenitor cells in chronic kidney disease. Nephrol. Dialysis Transplant. 25, 341–346 (2010).
Groot, Kde et al. Uremia causes endothelial progenitor cell deficiency. Kidney Int. 66, 641–646 (2004).
Issa, N. & Braun, W. E. Immunosuppression for Renal Transplant Patients and Common Medical Problems in Renal Transplantation. Available at http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/nephrology/immunosuppression-and-renal-transplant/ (2010).
Tendron-Franzin, A. et al. Long-term effects of in utero exposure to cyclosporin A on renal function in the rabbit. JASN 15, 2687–2693 (2004).
Zheng, S. et al. Pharmacokinetics of tacrolimus during pregnancy. Therapeutic drug Monit. 34, 660–670 (2012).
Freriksen, J. J. M. et al. Placental disposition of the immunosuppressive drug tacrolimus in renal transplant recipients and in ex vivo perfused placental tissue. Eur. J. Pharm. Sci. 119, 244–248 (2018).
Chang, T.-Y. et al. Dysregulation of endothelial colony-forming cell function by a negative feedback loop of circulating miR-146a and −146b in cardiovascular disease patients. PloS ONE 12, e0181562 (2017).
Kang, M. & Thébaud, B. Stem cell biology and regenerative medicine for neonatal lung diseases. Pediatr. Res 83, 291–297 (2018).
Baker, C. D. et al. Endothelial colony-forming cells from preterm infants are increased and more susceptible to hyperoxia. Am. J. Respir. Crit. Care Med. 180, 454–461 (2009).
Ligi, I. et al. A switch toward angiostatic gene expression impairs the angiogenic properties of endothelial progenitor cells in low birth weight preterm infants. Blood 118, 1699–1709 (2011).
Hu, Z. et al. Low-dose aspirin promotes endothelial progenitor cell migration and adhesion and prevents senescence. Cell Biol. Int. 32, 761–768 (2008).
Chen, T.-G., Chen, J.-Z. & Xie, X.-D. Effects of aspirin on number, activity and inducible nitric oxide synthase of endothelial progenitor cells from peripheral blood. Acta Pharmacol. Sin. 27, 430–436 (2006).
Dinelli, M. I. S., Ono, E., Viana, P. O., Dos Santos, A. M. N. & Moraes-Pinto, M. Ide Growth of children born to renal transplanted women. Eur. J. Pediatrics 176, 1201–1207 (2017).
Bachmann, F. et al. Pregnancy following kidney transplantation - impact on mother and graft function and focus on childrens’ longitudinal development. BMC Pregnancy Childbirth 19, 376 (2019).
Acknowledgements
The authors thank the staff of the Division of Obstetrics at Hannover Medical School for support in recruiting participants and collecting blood samples, and Katja Richter for technical assistance. The authors thank the Claudia von Schilling Foundation for provision of the xCelligence instrument.
Funding
This work was supported by a grant from the German Federal Ministry of Education and Research (reference number: 01EO1302). Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
N.M. contributed to the conceptualization and design of the study, research performance, data collection, data analysis, and drafted the initial manuscript. T.H.V. and L.B. contributed to the design of the study and the data collection. B.S.H. contributed to the design of the study. C.v.K contributed important research resources. F.v.V.H. contributed to the conceptualization and design of the study, contributed important research resources, and assisted in the manuscript preparation. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Category of study
Clinical study combined with basic science.
Ethics approval
The study was approved by the Institutional Review Board of Hannover Medical School (approval no. 1443-2012 and 504–2009).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Meyer, N., Vu, T.H., Brodowski, L. et al. Fetal endothelial colony-forming cell impairment after maternal kidney transplantation. Pediatr Res 93, 810–817 (2023). https://doi.org/10.1038/s41390-022-02165-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02165-x