Abstract
Background
Neonatal Resuscitation Program does not recommend placental transfusion in depressed preterm neonates.
Methods
Our objectives were to study the effect of delayed cord clamping (DCC) with ventilation for 5 min (DCCV, n-5), umbilical cord milking (UCM) without ventilation (n-6), UCM with ventilation (UCMV, n-6), early cord clamping followed by ventilation (ECCV, n-6) on red cell volume (RCV), and hemodynamic changes in asphyxiated preterm lambs. Twenty-three preterm lambs at 127–128 days gestation were randomized to DCCV, UCM, UCMV, and ECCV. We defined asphyxia as heart rate <100/min.
Results
The UCMV had the highest neonatal RCV as a percentage of fetoplacental volume compared to the other groups (UCMV 85.5 ± 10%, UCM 72 ± 10%, ECCV 65 ± 14%, DCCV 61 ± 10%, p < 0.01). The DCCV led to better ventilation (66 ± 1 mmHg) and higher pulmonary blood flow (75 ± 24 ml/kg/min). The carotid flow was significantly higher in UCM without ventilation. The fluctuations in carotid flow with milking were 25 ± 6% higher from baseline during UCM, compared to 6 ± 3% in UCMV (p < 0.01).
Conclusions
Cord milking with ventilation led to higher RCV than other interventions. Ventilation during cord milking reduced fluctuation in carotid flow compared to UCM alone. DCCV led to better ventilation and pulmonary blood flow but did not increase RCV.
Impact
-
The best practice of placental transfusion in a depressed preterm neonate remains unknown.
-
Ventilation with an intact cord improves gas exchange and hemodynamics in an asphyxiated preterm model.
-
Cord milking without ventilation led to lower red cell volume but higher carotid blood flow fluctuations compared to milking with ventilation.
-
Our data can be translated to bedside and could impact preterm resuscitation.
Similar content being viewed by others
Introduction
International Liaison Committee on Resuscitation suggests at least a 30-s deferral in cord clamping during preterm birth not needing immediate resuscitation.1 This recommendation is based on the potential benefits of fewer blood transfusions, and lower risk of intraventricular hemorrhage (IVH), and a trend toward lower mortality in premature infants.2,3 Recently, a large randomized control trial (RCT) reported mortality data in preterm infants <30 weeks gestation between immediate and delayed cord clamping (DCC).4 Although mortality was lower in this study with DCC (6.4% vs. 9%) in unadjusted analysis (p = 0.03), it was not significant (p = 0.39) after post hoc adjustment for multiple secondary outcomes.5
There is insufficient evidence for optimal cord management in preterm infants with low heart rate (HR) needing immediate resuscitation. Two approaches to cord management—ventilation with an intact cord or umbilical cord milking (UCM)—are being evaluated for infants requiring immediate resuscitation. A translational study in preterm lambs showed that, when ventilation was provided before cord clamping, there was a significant improvement in cerebral and systemic oxygenation.6 A feasibility study showed that DCC for 5 min improved blood pressure and cerebral oxygenation in term neonates at risk of resuscitation.7
Studies evaluating UCM in preterm infants have demonstrated both benefits and risks. In an RCT of infants <32 weeks, UCM (20 cm stripped three times) led to higher HR, oxygen saturation, and lower oxygen requirement in the first 10 min.8 Preterm neonates who had undergone UCM had better cognitive and language scores at 22–26 months when compared to DCC.9 In infants requiring resuscitation, milking the cord and allowing it to refill before stripping each time could aid in placental transfusion without delay in initiating resuscitative measures. The UCM in a preterm lamb model had shown fluctuations in carotid pressure and flow, especially when placental refill was not practiced.10 Katheria et al., in an RCT of preterm infants, have demonstrated severe IVH in extremely preterm infants with UCM when compared to DCC.9
The cord management strategies (DCC or UCM) in asphyxiated preterm neonatal models with perinatal metabolic acidosis requiring immediate resuscitation have not been evaluated. Our objectives were to study the effect of early cord clamping followed by ventilation (ECCV), ventilation with DCC for 5 min (DCCV), umbilical cord milking with placental refill (UCM), UCM with the placental refill, and concomitant ventilation (UCMV) in the immediate resuscitation phase in an asphyxiated preterm model on red cell volume (RCV), gas exchange, and cerebral and pulmonary hemodynamics.
Methods
Time-dated pregnant ewes at 127–128 days gestation (term ~145–150 days) were studied as per ARRIVE guidelines after approval by the Institutional Animal Care and Use Committee of the University at Buffalo. The pregnant ewes did not receive antenatal steroids. As described previously, the preterm lamb fetus was instrumented while on placental circulation and with the ewe under general anesthesia.11 A jugular venous line is placed for access and blood draw. The right carotid artery is catheterized for pressure monitoring and for arterial blood gas draws. Blood flow transducers are placed to monitor the left common carotid blood flow, left pulmonary artery blood flow, and ductal flow. The lambs were intubated with a cuffed endotracheal tube (ETT), and lung liquid was passively drained by gravity. ETT was occluded before delivery to prevent air entry during gasping. Asphyxia was induced by cord occlusion until the HR dropped below 90 beats per minute (bpm). We defined asphyxia in our group as a HR < 100 bpm. To ensure that the preterm lambs HR remained <100 bpm, we targeted an HR of 90 bpm. After asphyxia, the lambs were randomized to one of the four protocols: (i) ECCV, (ii) DCCV, (iii) UCM, and (iv) UCMV.
-
i.
ECCV—Following asphyxia by cord occlusion, the cord was clamped and ventilation was initiated with change in inspired oxygen targeting preductal saturations as per neonatal resuscitation program (NRP) recommendations.
-
ii.
DCCV—Following asphyxia, compression of the umbilical cord was released and ventilation was initiated with an intact cord for 5 min before clamping the cord.
-
iii.
UCM—Following asphyxia, the umbilical cord compression was released and milking was performed by stripping the umbilical cord four times and allowing the cord to fill with the placental flow by pinching the cord on the fetal side to prevent blood from returning from the fetus. Each stripping of the cord and allowing the cord to refill with placental blood took approximately 10 s. No ventilation was provided for approximately 40 s. The cord was clamped, and ventilation was initiated.
-
iv.
UCMV—Following asphyxia, compression of the umbilical cord was released, and ventilation was initiated, followed by milking for 40 s (as described above but with ventilation), after which the cord was clamped.
RCV estimation was done based on a previous study by Strauss et al.12 Circulating RCV was measured using biotinylated red blood cells (RBCs) in the neonatal lamb expressed as a percentage of fetoplacental volume to assess the magnitude of placental transfusion.12 Low- and high-density biotinylated RBCs were used to estimate fetoplacental baseline volume and the newborn volume after the intervention. The effect of the initial intervention was measured at 2 h (at the end of the study period) to allow for postnatal equilibration. During this period, the intravenous fluids were infused at 120 ml/kg/day, and similar volumes of blood were drawn for blood gas sampling in all groups.
Left carotid, left pulmonary, and ductal flows and blood pressures were continuously monitored throughout the period of observation. In addition, during cord milking, the fluctuations in carotid and pulmonary blood flows with each milk from baseline were measured and represented as a percentage change (fluctuation with cord milking = increase in flow in ml/min at the peak during milking ÷ baseline flow before milking).
Right carotid arterial blood gases were collected at asphyxia, 1, 2, and 5 min of ventilation. The oxygen was titrated based on saturations (SpO2) targets as recommended by NRP. Supplemental O2 was initiated at 21% and titrated up by 10% every 30 s to achieve the desired targets. The positive pressure ventilation (PPV) was initiated with peak inspiratory pressures of 30 cmH2O with a peak end-expiratory pressure of 5 cmH2O targeting tidal volumes 8 ml/kg. The pressures were adjusted in order to achieve target tidal volumes every 15 s.
Sample size estimation
The sample size for this study was based on a difference in the percentage of RCV between the four groups. Based on our preliminary study, to see a difference in RCV as a percentage of fetomaternal volume between four groups, with an average difference of 15% and a standard deviation of 10% in RCV, with coefficients of 1, −1, −1, and −1 (ECCV as control group), 6 lambs in each group by analysis of variance (ANOVA) were needed for this study, with a power of 0.92 and a type I probability of 0.05 (total n = 24). This sample size has adequate power to detect a 20 ml/kg/min difference in pulmonary blood flow, a 5 ml/kg/min difference in carotid blood flow, and a 15 mmHg difference in partial pressures of carbon dioxide (PaCO2) between the groups.
Statistical analysis
Kolmogorov–Smirnov test was done to determine the parametric/non-parametric distribution of the data, and the analysis was done accordingly. For all four groups, we used two-way repeated-measures ANOVA for continuous parametric variables. Post hoc tests were done using the Bonferroni correction. The differences were for the overall profile and not the specific time points. Categorical variables were analyzed using the multiple group chi-square test.
UCM and UCMV—since the comparisons between the fluctuations were only done during milking, we used the unpaired t test. The significance was set at a probability of <5%.
Results
A total of 23 preterm lambs were evaluated in this study. They were randomized as follows: six in the ECCV, UCM, and UCMV groups and five in the DCCV group (due to arrest in one lamb). The characteristics of the lambs randomized are shown in Table 1. Following asphyxiation by cord occlusion, preterm lambs in all the groups had similar combined respiratory and metabolic acidosis (Table 2).
After initiating PPV, the HRs by 1 min were not significantly different (Fig. 1a).
a shows the heart rate (HR) at asphyxia and 1 min in each group. The HRs were not different between the groups at asphyxia and at 1 min. b Neonatal blood volume as a percentage of fetoplacental volume; asterisk (*) represents statistical significance p < 0.01. c Cumulative inspired oxygen concentration during 1–5 min in the four groups of lambs. The horizontal dotted line represents 50% inspired oxygen.
RCV and hemoglobin
The post-intervention neonatal RCVs between each group as a percentage of fetomaternal volume are shown in Fig. 1b. The UCMV had the highest RCV compared to the other groups.
Oxygenation and ventilation
After asphyxiation and initial resuscitation with 21% O2 and titration, 75% SpO2 was achieved by 5 min in 3/5 (60%) of DCCV, and 1/6 (17%) of UCMV, and none in the ECCV and UCM groups. The cumulative oxygen requirement during 5 min of resuscitation is shown as box plots (Fig. 1c). In the first 5 min, 75% of lambs in the DCCV group needed <50% oxygen, which was significantly different from the other groups. The arterial oxygenation was not statistically different between the groups (Fig. 2a). Carbon dioxide levels were the lowest with DCCV, while UCM without ventilation had significantly higher PaCO2 levels (Fig. 2b). The pH significantly improved in the DCCV group and was significantly higher at 5 min than the rest (Fig. 2c).
Pulmonary and carotid blood flow
Pulmonary blood flow increased with ventilation in all lambs (Fig. 3a). The pulmonary blood flows were lowest in the UCM group. DCCV had significantly higher pulmonary flows when compared to other groups. The carotid blood flow was significantly higher in the UCM group compared to other groups (Fig. 3b). Ductal flows in the fetal lambs were right to left (pulmonary-to-systemic circulation). At 5 min, the ductal shunting reversed left to right (systemic-to-pulmonary circulation) in the DCCV and UCMV groups but not in ECCV and UCM groups (Fig. 3c). The flows across the ductus were not significantly different. The mean blood pressures were similar in all the groups (Fig. 3d).
Flows during UCM
Fluctuations in carotid and pulmonary blood flows during milking were assessed with (UCMV) and without ventilation (UCM) (Fig. 4) and expressed as a percentage of baseline flows during the four cord-stripping maneuvers. The fluctuations observed in carotid blood flow (UCMV 6.9 ± 2.6% vs. UCM 24.3 ± 6.1%, respectively, p < 0.0001) and pulmonary blood flow (UCMV 4.8 ± 4.5% vs. UCM 22.7 ± 13.6%, respectively, p = 0.023), were significantly higher when umbilical cord milking was performed without ventilation from baseline, compared to UCM with ventilation (Fig. 5).
Discussion
Placental transfusion facilitates transition at birth by three distinct mechanisms—volume effect by adding circulating blood volume to the neonate, oxygenation by providing umbilical venous return (with SO2 ~80–85%), and circulatory stabilization by contributing to left ventricular preload.13 Initiating PPV in a depressed preterm infant is the most important step to facilitate transition.14 In term infants, with the initial cries, the lungs take over the function of gas exchange from the placenta. As a result, the pulmonary vascular resistance (PVR) decreases and pulmonary blood flow increases, and the shunt across the ductus is from systemic to pulmonary circulation.15,16 However, in a depressed premature neonate, acidosis, hypoxia, poor respiratory effort, immature lung architecture, and function may delay the transition. A meta-analysis by Oei et al. has shown that preterm neonates who do not achieve saturations of >80% by 5 min and bradycardic are at risk of IVH and death.17 In another retrospective analysis, preterm neonates remaining bradycardic for 5 min (with HR < 60 bpm) with SpO2 < 80% were at 18 times higher risk of mortality.18 Thus, avoiding delays in ventilation with simultaneous placental transfusion in preterm neonates may potentially lead to better outcomes.
Two approaches have been suggested to avoid ventilation delays due to placental transfusion. One approach is to provide PPV during DCC, and the second approach is to accelerate placental transfusion by cord milking. Previous studies in preterm lambs have shown that ventilation during DCC (physiological-based cord clamping) provided circulatory stability but did not lead to a net transfer of blood volume from the placenta to the neonate.10 Cord milking would be a logical step to provide additional blood volume immediately after birth and continue with resuscitation but is associated with fluctuations in cerebral blood flow and IVH in preterm.9 In this study, we evaluated cord milking with placental refill with simultaneous ventilation and demonstrated significantly higher transfusion of RCV and minimized fluctuations in cerebral blood flow with milking.
Increasing the transfer of placental blood volume to the neonate is a hematologic benefit of placental transfusion. Blank et al. have elegantly shown that, in a non-asphyxiated preterm model, milking the cord eight times with placental refill significantly increased the blood volume.10 However, DCC with ventilation did not result in a net transfer of blood to the non-asphyxiated neonatal lamb.10 Our study confirmed these findings in an asphyxiated preterm lamb model (Fig. 1). In addition, clinical trials among preterm infants predominantly delivered by cesarean section demonstrate higher hemoglobin with UCM but not with DCC, consistent with this finding.19,20
The second benefit of placental transfusion is the delivery of oxygenated umbilical venous blood with the placenta continuing to serve as a site of gas exchange.6,21,22,23,24,25,26 Lambs in the DCCV group had the highest pH, lowest PaCO2, and less inspired oxygen load. This approach of ventilation before clamping the cord is known as physiological-based cord clamping.6 It has been associated with higher cerebral oxygen saturations than UCM in non-asphyxiated preterm lambs.10 In non-asphyxiated10 and asphyxiated preterm lambs, physiological-based cord clamping (DCCV) appears to be the most effective technique to improve oxygenation and ventilation due to “dual-site” (placenta and lungs) gas exchange and improved pulmonary blood flow. Milking with placental refill without ventilation (UCM) deprives the lamb of this benefit and is associated with high PaCO2, low pH, and low pulmonary blood flow. During milking (UCMV), ventilation did not significantly improve pulmonary blood flow and did not appear to have the same benefits as physiological-based cord clamping. However, the benefits of providing additional oxygen-carrying capacity with RBC volume transfused with UCMV will potentially benefit tissue oxygen delivery and could be more clinically relevant in extremely preterm infants than just increasing pulmonary blood flow by DCCV.27
The third benefit of placental transfusion is circulatory stabilization. In preterm neonates, excessive fluctuations and rapid increases in cerebral blood flow can potentially be associated with IVH.28 Studies in non-asphyxiated preterm lambs have suggested that milking of the cord could lead to rapid increase and fluctuations in carotid blood flow.10 Stenning et al. demonstrated that infusion of blood to a non-asphyxiated preterm lamb (10 ml/kg of blood over 90 s) led to a transient increase in carotid blood flow and arterial pressure, and these values decreased after ventilation onset.29 These findings suggest that increased circulatory volume due to blood infusion without simultaneous ventilation can lead to abrupt increases in cerebral blood pressure and flow. Similar increases in carotid blood flow were observed following UCM.10
Our study found decreased fluctuations in both carotid and pulmonary blood flow when the cord was milked with placental refill and ventilation compared to UCM alone. The UCM with ventilation led to similar carotid flows to DCCV and ECCV, with a change in the direction of ductal flows from the left to right at 5 min. We speculate that ventilation of the lung and decreased PVR minimized fluctuations in carotid blood flow. The fluctuations in both carotid and pulmonary blood flow from the baseline when the cord was milked with placental refill but without ventilation was similar to a previous study in a non-asphyxiated preterm ovine model (Fig. 4).10
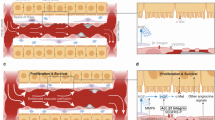
Delaying the ventilation for 40 s until UCM was performed led to significantly higher carbon dioxide levels. In an asphyxiated preterm infant, higher CO2 levels and hemodynamic fluctuations incurred during milking of the umbilical cord could affect cerebral blood and be detrimental in premature neonates. In a post hoc analysis of a RCT, UCM (milking 3 times with 2 s refill from placenta) in preterm infants was associated with a higher rate of severe IVH compared to delayed umbilical cord clamping.30,31 With the available evidence from clinical and translational studies, UCM without ventilation does not demonstrate any benefit in an asphyxiated depressed preterm neonate without respiratory effort (except for a non-significant increase in blood volume) and could lead to further complications, such as severe IVH. In contrast, physiological-based cord clamping (DCCV) did not increase blood volume in our study but had better hemodynamics and gas exchange (Fig. 6).
Umbilical cord milking produces fluctuations in flow in the descending aorta. With the initiation of ventilation, there is increased pulmonary blood flow and a left-to-right ductal shunt. The left-to-right ductal shunt and increased pulmonary blood flow buffer these changes in flow, minimizing fluctuations in carotid blood flow compared to cord milking without ventilation (copyright Satyan Lakshminrusimha).
There are several limitations of our study. This model of asphyxiated preterm lamb is delivered by cesarean section under general anesthesia without uterine contractions. Inherent species differences exist since the ovine umbilical cord is short and has two arteries and two veins. The lung’s developmental stage in lambs is closer to human neonates than that of brain. Regarding instrumentation, a limitation is cannulation of the contralateral carotid artery that could have interfered with the common carotid artery blood flow measurement. To compare the milking with milking and ventilation, we did allow the cord to refill, and the entire procedure took around 40 s, which could be shorter in human neonates given the longer length of the umbilical cord. The hemodynamic advantages of continuous ventilation during DCC and UCM over the respective group, compared with early cord clamping and ventilation (ECCV), unfortunately, did not reach significance. The optimal clinical alternative to UCM would be DCC without ventilation or 40–60 s of DCC followed by ventilation. One limitation with the model of cord compression for asphyxia is that it takes a few seconds for the umbilical veins to open up (with improving HR) before placental transfusion is established. Following the release of the umbilical cord compression, a short delay in the clamping of the cord in this model could have worsened bradycardia and led to higher PaCO2 levels with no placental flow in the absence of ventilation. The initiation of ventilation improved the HR that led to the establishment of blood flows across the umbilical cord. Thus, the PaCO2 was significantly lower with ventilation. In the UCM group, milking was performed, allowing refill manually. While our study demonstrated the hemodynamic advantages of continuous ventilation during DCCV and UCMV over the respective group, the comparison with early cord clamping and ventilation (ECCV) unfortunately did not reach statistical significance. Nevertheless, our study provides mechanistic evidence of various approaches to cord management in asphyxiated bradycardic preterm neonates.
Conclusion
Various modalities of cord management have distinct physiological effects on transfusion volume, gas exchange, and transitional hemodynamics. In an asphyxiated preterm lamb model, physiological-based cord clamping by providing PPV with an intact umbilical cord (DCCV) led to better gas exchange and hemodynamics but did not increase RCV. Cord milking with ventilation (UCMV) led to higher blood cell volume and reduced fluctuation in carotid flow. Cord milking without ventilation did not significantly increase RCV but was associated with increased carotid flow fluctuations. Results of our study and the ongoing clinical trials of cord management in preterm infants will provide more evidence for clinical practice.
Data availability
Data presented in this manuscript will be available after complete analysis (2-hour data) is done on request.
References
Perlman, J. M. et al. Part 7: Neonatal Resuscitation: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation 132(Suppl 1), S204–S241 (2015).
Rabe, H., Diaz-Rossello, J. L., Duley, L. & Dowswell, T. Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. Cochrane Database Syst. Rev. 8, CD003248 (2012).
Rabe, H., Reynolds, G. & Diaz-Rossello, J. Early versus delayed umbilical cord clamping in preterm infants. Cochrane Database Syst. Rev. 4, CD003248 (2004).
Tarnow-Mordi, W. et al. Delayed versus immediate cord clamping in preterm infants. N. Engl. J. Med. 377, 2445–2455 (2017).
Katheria, A. C. Delayed cord clamping may not be beneficial in the premature infant. J. Pediatr. 196, 324–327 (2018).
Polglase, G. R. et al. Ventilation onset prior to umbilical cord clamping (physiological-based cord clamping) improves systemic and cerebral oxygenation in preterm lambs. PLoS ONE 10, e0117504 (2015).
Katheria, A. C. et al. Delayed cord clamping in newborns born at term at risk for resuscitation: a feasibility randomized clinical trial. J. Pediatr. 187, 313.e1–317.e1 (2017).
Katheria, A., Blank, D., Rich, W. & Finer, N. Umbilical cord milking improves transition in premature infants at birth. PLoS ONE 9, e94085 (2014).
Katheria, A. et al. A randomized clinical trial of umbilical cord milking vs delayed cord clamping in preterm infants: neurodevelopmental outcomes at 22-26 months of corrected age. J. Pediatr. 194, 76–80 (2018).
Blank, D. A. et al. Haemodynamic effects of umbilical cord milking in premature sheep during the neonatal transition. Arch. Dis. Child. Fetal Neonatal Ed. 103, F539–F546 (2018).
Chandrasekharan, P. et al. Effect of various inspired oxygen concentrations on pulmonary and systemic hemodynamics and oxygenation during resuscitation in a transitioning preterm model. Pediatr. Res. 84, 743–750 (2018).
Strauss, R. G. et al. Circulating RBC volume, measured with biotinylated RBCs, is superior to the Hct to document the hematologic effects of delayed versus immediate umbilical cord clamping in preterm neonates. Transfusion 43, 1168–1172 (2003).
Bhatt, S. et al. Delaying cord clamping until ventilation onset improves cardiovascular function at birth in preterm lambs. J. Physiol. 591(Pt. 8), 2113–2126 (2013).
Weiner, G. M. & Zaichkin, J. (eds) Textbook of Neonatal Resuscitation (NRP) 7th edn (American Academy of Pediatrics, 2016).
Lakshminrusimha, S. & Jobe, A. H. Baby’s first cries and establishment of gas exchange in the lung. Am. J. Respir. Crit. Care Med. 204, 11–13 (2021).
Tingay, D. G. et al. Imaging the respiratory transition at birth: unravelling the complexities of the first breaths of life. Am. J. Respir. Crit. Care Med. 204, 82–91 (2021).
Oei, J. L. et al. Outcomes of oxygen saturation targeting during delivery room stabilisation of preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 103, F446–F454 (2017).
Kapadia, V. et al. Outcomes of delivery room resuscitation of bradycardic preterm infants: a retrospective cohort study of randomised trials of high vs low initial oxygen concentration and an individual patient data analysis. Resuscitation 167, 209–217 (2021).
March, M. I., Hacker, M. R., Parson, A. W., Modest, A. M. & de Veciana, M. The effects of umbilical cord milking in extremely preterm infants: a randomized controlled trial. J. Perinatol. 33, 763–767 (2013).
Katheria, A. C., Truong, G., Cousins, L., Oshiro, B. & Finer, N. N. Umbilical cord milking versus delayed cord clamping in preterm infants. Pediatrics 136, 61–69 (2015).
Andersson, O. et al. Intact cord resuscitation versus early cord clamping in the treatment of depressed newborn infants during the first 10 min of birth (Nepcord III) - a randomized clinical trial. Matern. Health Neonatol. Perinatol. 5, 15 (2019).
Chandrasekharan, P. et al. Resuscitation with an intact cord enhances pulmonary vasodilation and ventilation with reduction in systemic oxygen exposure and oxygen load in an asphyxiated preterm ovine model. Children 8, 307 (2021).
Lakshminrusimha, S., Vali, P., Chandrasekharan, P., Rich, W. & Katheria, A. Differential alveolar and systemic oxygenation during preterm resuscitation with 100% oxygen during delayed cord clamping. Am. J. Perinatol. https://doi.org/10.1055/s-0041-1730362 (2021).
Padilla-Sanchez, C. et al. Delayed vs immediate cord clamping changes oxygen saturation and heart rate patterns in the first minutes after birth. J. Pediatr. 227, 149.e1–156.e1 (2020).
Rudolph, A. M. & Heymann, M. A. Cardiac output in the fetal lamb: the effects of spontaneous and induced changes of heart rate on right and left ventricular output. Am. J. Obstet. Gynecol. 124, 183–192 (1976).
Yigit, B., Tutsak, E., Yildirim, C., Hutchon, D. & Pekkan, K. Transitional fetal hemodynamics and gas exchange in premature postpartum adaptation: immediate vs. delayed cord clamping. Matern. Health Neonatol. Perinatol. 5, 5 (2019).
Katheria, A. C., Brown, M. K., Rich, W. & Arnell, K. Providing a placental transfusion in newborns who need resuscitation. Front. Pediatr. 5, 1 (2017).
Chawla, S., Chock, V. Y. & Lakshminrusimha, S. Intraventricular hemorrhage and white matter injury: is persistent cerebral desaturation a missing link? Pediatr. Res. 89, 727–729 (2021).
Stenning, F. J. et al. Transfusion or timing: the role of blood volume in delayed cord clamping during the cardiovascular transition at birth. Front. Pediatr. 7, 405 (2019).
Katheria, A. et al. Association of umbilical cord milking vs delayed umbilical cord clamping with death or severe intraventricular hemorrhage among preterm infants. JAMA 322, 1877–1886 (2019).
Rabe, H. & Andersson, O. Maternal and infant outcomes after different methods of umbilical cord management. JAMA 322, 1864–1865 (2019).
Funding
This work was financially supported by R01HD104909, R03HD096510, K12 HL138052, AAP NRP grant, Zoll Foundation grant, and University at Buffalo—Dr. Henry C. and Bertha H. Buswell Grant to P.C.; R03HD104062 to M.R.; and R01HD072929 to S.L.
Author information
Authors and Affiliations
Contributions
P.C.—conceptualization, methodology, data acquisition and analysis, interpretation, writing—original draft, critically reviewing, and editing. S.G., C.K., J.H., L.N., N.B., J.N., D.S., M.B., M.R.—data acquisition, extraction, critical reviewing, and manuscript editing. S.L.—methodology, data interpretation, critically reviewing, editing, and provided illustration. All authors approved the final version for publication.
Corresponding author
Ethics declarations
Competing interests
S.L. is a neonatal resuscitation program steering committee member. All other authors report no conflicts of interest. The research reported here is not endorsed by the funding institutions or the neonatal resuscitation program.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chandrasekharan, P., Gugino, S., Koenigsknecht, C. et al. Placental transfusion during neonatal resuscitation in an asphyxiated preterm model. Pediatr Res 92, 678–684 (2022). https://doi.org/10.1038/s41390-022-02086-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02086-9
This article is cited by
-
From “A family of NICU graduates” to “A family of four”
Pediatric Research (2022)