Abstract
Background
Hypoxic–ischemic encephalopathy (HIE) is a devastating disease with lifelong disabilities. Hypothermia is currently the only treatment. At term, the neonatal cerebellum may be particularly vulnerable to the effects of HIE. At this time, many developmental processes depend on lipid raft function. These microdomains of the plasma membrane are critical for cellular signaling and axon extension. We hypothesized that HIE alters the protein content of lipid rafts in the cerebellum.
Methods
Postnatal day (PN) 10 animals, considered human term equivalent, underwent hypoxic–ischemic (HI) injury by a right carotid artery ligation followed by hypoxia. For some animals, LPS was administered on PN7, and hypothermia (HT) was conducted for 4 h post-hypoxia. Lipid rafts were isolated from the right and left cerebella. The percent of total L1 cell adhesion molecule in lipid rafts was determined 4 and 72 h after hypoxia.
Results
No sex differences were found. HI alone caused significant increases in the percent of L1 in lipid rafts which persisted until 72 h in the right but not the left cerebellum. A small but significant effect of LPS was detected in the left cerebellum 72 h after HI. Hypothermia had no effect.
Conclusions
Lipid rafts may be a new target for interventions of HIE.
Impact
-
This article investigates the effect of neonatal exposure to hypoxic–ischemic encephalopathy (HIE) on the distribution of membrane proteins in the cerebellum.
-
This article explores the effectiveness of hypothermia as a prevention for the harmful effects of HIE on membrane protein distribution.
-
This article shows an area of potential detriment secondary to HIE that persists with current treatments, and explores ideas for new treatments.
Similar content being viewed by others
Introduction
Hypoxic–ischemic encephalopathy (HIE), characterized by death or impaired neurodevelopment secondary to insufficient oxygen or blood flow to the brain, affects 1–3 out of every 1000 infants.1,2 Clinical outcomes are variable, as the timing of the ischemic event and concomitant morbidities can change the course of the injury and effectiveness of interventions.3 In moderate to severe cases of HIE, for infants born between 36 and 42 weeks’ gestation, hypothermia is the standard clinical intervention,4 increasing likelihood of survival (reviewed in refs. 5,6) Hypothermia has been shown to suppress apoptosis and inflammation after HIE.7,8,9 However, both the efficacy and application of hypothermia is limited. Its effectiveness falls drastically as the time between birth and initiation of cooling increases and is less likely to produce observable neuroprotection when pre-existing inflammation is present.10,11
The majority of studies examining cerebellar outcomes after hypoxia–ischemia (HI) examined very early postnatal ages to model premature injury.12,13,14 In the rodent, the first postnatal week is a period of robust cell genesis and migration in the cerebellum.15,16,17 Proliferation, migration, and refinement of circuitry continues through the first two to three weeks in the rodent, and through the first several months of postnatal development in the human.18 This long developmental time course is hypothesized to increase the vulnerability of the cerebellum to disorders of development and perinatal brain injury (reviewed in refs. 19,20) The most established model of HIE in rodents is the Levine method, which consists of a unilateral carotid artery ligation followed by hypoxia.21,22 This approach produces an injury most obvious in the cerebral hemisphere ipsilateral to the carotid artery ligation, where the study of injury mechanisms has largely focused.23,24 Little is known regarding the sensitivity of the cerebellum to hypoxia–ischemia (HI), or its response to hypothermia with or without inflammation.
L1 is a member of the immunoglobulin (Ig) superfamily and a cell adhesion molecule that directly regulates axon growth during brain development.25,26,27 Our group has established that perinatal insults can increase the distribution of L1 to lipid rafts in developing the cerebellum, disrupting normal neurite outgrowth and cerebellar function.28,29,30 Lipid rafts are highly organized microdomains of the plasma membrane, composed of sphingolipids and cholesterol in the outer exoplasmic leaflet, and bound to phospholipids and cholesterol in the inner cytoplasmic leaflet of the lipid bilayer.31 These dynamic microdomains are enriched with signaling molecules and promote interactions of growth factors and signaling pathways.25 Association of L1 with lipid rafts is tightly restricted to specific phases of brain development. In mouse cerebellum, L1 is detected in lipid rafts only between postnatal days 3–28, comparable to 23 gestation weeks up to 11 years of age in humans.32,33 By adulthood, L1 is no longer associated with lipid rafts at a detectable level.32 When L1 association with lipid rafts is dysregulated, L1 cannot guide neurite elongation and migration of neuronal precursors.28 The developmental regulation of L1 and its association with lipid rafts might indicate when brain regions are vulnerable to injury.
Lipid rafts play a key role in signal transduction through the cell membrane in most cell types. Lipopolysaccharide (LPS) is a potent inducer of an immune response. It is a structural component of most gram-negative bacteria and its effects are mediated by its interaction with toll-like receptor 4 (TLR-4).34,35 Activation of the TLR-4 receptor causes its relocation to lipid rafts in macrophages and glial cells, where inflammatory signal transduction pathways are initiated.36,37 LPS-induced inflammation sensitizes HI brain injury both acutely and after systemic indications of infections have resolved38,39,40 and reduces the efficacy of hypothermia.10,41,42 Whether TLR-4 activation also influences the redistribution of L1 to lipid rafts in developing neurons in the context of neonatal HI is unknown. In the adult brain, focal stroke injury in the cerebrum produces a reduction of blood and oxygen in the cerebellum contralateral to the injured area of the cerebrum, a phenomenon known as crossed-cerebellar diaschisis.43 Here, we examined the effect of HI on L1 distribution in lipid rafts in the right (ipsilateral to ligation) and left (contralateral to ligation) cerebellum in the P10 rat pup to model near-term or term birth.44 We hypothesized that HI would disrupt L1 trafficking through lipid rafts in the cerebellum of term equivalent rat pups. We further determined the effect of inflammation and hypothermia. If inflammation exacerbates the redistribution of L1 to lipid rafts in neurons or changes the ability of hypothermia to normalize L1 distribution in lipid rafts, this would suggest a mechanism by which inflammation changes the progression of HI and response to intervention. Our results confirm that the ipsilateral cerebellum was more robustly affected by HI, and that neither inflammation nor hypothermia significantly changed L1 distribution in the right cerebellum. These effects persisted for 72 h.
Methods
Animals
All studies were approved by the University of Maryland School of Medicine Institutional Animal Care and Use Committee. Timed pregnant females arrived at the animal facility at the University of Maryland, Baltimore where the dams were permitted to deliver naturally. All dams were acquired from the Charles River. The day of birth was recorded and considered postnatal day 1 (PN1). On PN7, pups were randomly assigned to receive an intraperitoneal injection of LPS (200 mg/kg) or saline (Fig. 1). On PN10, the dam and pups were weighed and randomly assigned to experimental condition. Pups to be subjected to hypoxia–ischemia (HI) were anesthetized with isoflurane. The right carotid artery was isolated, ligated, and severed. Surgery times were 5 min or less. Sham-operated controls (Sham) were also anesthetized, received a small incision and a suture. Sham-operated control pups did not receive LPS or hypothermia. The carotid artery was not subject to manipulation. All pups were permitted to recover in normothermic conditions (37 °C) for 25 min. Following this recovery period, pups were returned to the dam for 1 h. HI pups were then placed in jars for hypoxia. Jars were placed in a water bath, each containing 1 or 2 pups. The jars were sealed and 8% O2 (92% N2) was flushed through the jars for 1 h. Sham-operated pups were removed from the dam for the same intervals and maintained in normothermia and normoxia for these intervals. At the end of hypoxia, pups were immediately placed in a large open jar at room air in normothermia or hypothermia (32 °C). Hypothermia commenced for 4 h. Following 4 h of hypothermia or normothermia, pups remained in the normothermia condition for 1 h and were then returned to the dam (Fig. 1). The number of pups in each group collected at the 4 h post-HI timepoint was: Sham: 6 males, 4 females; HI: 6 males, 7 females; HI + Hypo: 5 males, 5 females; LPS + HI: 4 males, 6 females; LPS + HI + Hypo: 4 males, 6 females. The number of pups in each group collected 72 h after HI was: Sham: 3 males, 3 females; HI: 4 males, 3 females; HI + Hypo: 3 males, 4 females; LPS + HI: 3 males, 3 females; LPS + HI + Hypo: 3 males, 3 females.
Tissue collection
The pups were decapitated, the cerebellum was collected and divided into right and left sides immediately after hypothermia (4 h, Fig. 1) or 72 h after the end of hypoxia. Cerebella were immediately frozen in liquid nitrogen. At the time of tissue collection, each brain was judged for the severity of injury with the following categories: 0 = nothing obvious; 1 = right cerebral hemisphere smaller with no visible infarct; 2 = small visible infarct in the right cerebral hemisphere; 3 = moderate infarct, comprising >50% of the hemisphere; 4 = severe infarct, comprising >50% of the hemisphere. Scores were recorded for tissue collected 72 h after HI only, as the injury is not visible without histological analysis 4 h after HI.
Tissue preparation
Each cerebellum was homogenized in ice-cold Tris-buffered saline containing 0.5% Triton X-100, 10 μM sodium vanadate, 2 μM aprotinin, 100 pM cypermethrin, phosphatase inhibitor cocktail I (Sigma-Aldrich), and phosphatase inhibitor cocktail II (Sigma-Aldrich). Homogenates were kept on ice for 30 min and then centrifuged at 13,000 × g for 10 min at 4 °C, and supernatants were collected. Protein was determined using the BCA Protein Assay Kit (ThermoFisher Scientific, Waltham, MA, USA).
Lipid raft isolation
All procedures were carried out on the ice and all buffers were kept at 4 °C for the duration of the procedure. One ml of supernatant was mixed with an equal amount of 80% sucrose solution and placed in a 12 ml ultracentrifuge tube. A discontinuous 5-35-40% sucrose gradient was formed by layering 4 ml of 35% (w/v) sucrose solution on top of the 2 ml homogenate, followed by 4 ml 5% (w/v) sucrose solution. The samples were centrifuged at 180,000 × g for 24 h at 4 °C in a Beckman SW41 rotor. Ten 1 ml fractions were collected from the top of each gradient. An aliquot from each fraction was analyzed by immunoblot analysis for the presence of GM1 ganglioside to determine fractions containing lipid rafts. All fractions containing GM1 ganglioside were combined into a lipid raft pool. All remaining fractions were combined in a non-lipid raft pool. The pools were made equivolume and equal volumes were taken for immunoblot analysis.
Antibodies and materials
Goat polyclonal anti-neural cell adhesion molecule L1 polyclonal antibody to the cytoplasmic domain of L1 (anti-L1CD) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal antibody to mouse IgG, and rabbit polyclonal antibody to mouse IgG (H + L) conjugated to hydrogen peroxidase were obtained from Jackson Immuno-Research (West Grove, PA, USA). Horseradish peroxidase (HRP)-conjugated cholera toxin B subunits (CTXB) were purchased from Sigma.
Immunoblotting
Samples were mixed with lithium dodecyl sulfate sample buffer (Invitrogen, Grand Island, NY, USA) and boiled for 5 min. Boiled samples were electrophoresed in 4–15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to a polyvinylidene difluoride membrane. The membranes were blocked for 1 h with 5% nonfat milk and then incubated with a primary antibody overnight at 4 °C. Blots were incubated in a horseradish peroxidase-conjugated secondary antibody against the primary at room temperature for 1 h. The blots were developed using the enhanced chemiluminescence system (GE Healthcare, Piscataway, NJ, USA) and visualized using Fluorchem HD2 imaging system (Alpha Innotech, Santa Clara, CA, USA). Where indicated, membranes were stripped and re-blotted for beta-actin to confirm equal loading. Densitometry was performed using the NIH Image J program (National Institutes of Health, Bethesda, MD, USA) to quantitate optical density to calculate the proportion of L1 in lipid rafts.
Statistical analysis
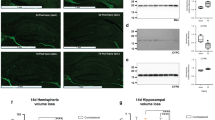
The proportion of L1 in the lipid raft pool was calculated as the pixels in the western blot band for L1 in the lipid raft lane divided by the total pixels in the L1 band of the lipid raft lane and the non-lipid raft lane (Fig. 2). For each side of the cerebellum, one-way ANOVA was conducted to determine the presence of between-group differences. A significant result was then followed up by Tukey’s pairwise comparison. The Tukey inference of p < 0.05 was considered statistically significant. Paired samples t-tests were used to compare the left and right cerebella within each experimental group. The non-parametric Spearman’s rho correlation was used to determine whether there was a relationship between the magnitude of injury rankings and proportion of L1 in lipid rafts in tissue collected 72 h after HI. We further analyzed the effect of time using independent t-tests for each group.
Due to limitations of the ultracentrifuge on preparing lipid rafts, each immunoblot is limited to 3 animals. Shown here are male animals treated with lipopolysaccharide (LPS), and hypoxia/ischemia (HI) and hypothermia (Hypo), HI alone, or Sham control. Left and right indicate samples from the left side or the right side of the cerebellum. Lipid rafts (L) were isolated by low detergent extraction and sucrose density gradient ultracentrifugation. Fractions containing lipid rafts (L) were pooled as were the non-lipid raft fractions (N) and equivolumes of the pools were run by SDS-gel electrophoresis and immunoblotted for L1.
Results
Analysis of sex as a variable did not detect any differences or interactions of sex at either the 4 or 72 h timepoint (largest F(1,24) = 2.25, p < 0.14). Therefore, results for males and females were combined for statistical analysis.
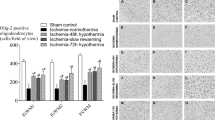
At the initial timepoint (4 h after the end of hypoxia), one-way ANOVA confirmed a significant effect of group in both the right, F(4,49) = 76.131, p < 0.0001, and left sides of the cerebellum, F(4,49) = 17.29, p < 0.0001. HI significantly increased the proportion of L1 in lipid rafts in both the right, p < 0.0001 (Fig. 3a), and left, p < 0.018 (Fig. 3b), cerebellum compared to sham-operated control pups (Fig. 3). In the right cerebellum, HI increased %L1 regardless of LPS or hypothermia (Hypo) in all groups compared to the sham group, all ps < 0.0001. There were no significant differences between HI + Hypo and HI, p > 0.05, or LPS + HI and HI, p > 0.05. Hypothermia also did not significantly change the %L1 in lipid rafts in pups treated with LPS (LPS + HI vs LPS + HI + Hypo, p > 0.5). In the left cerebellum, hypothermia did not significantly reduce %L1 in lipid rafts compared to HI alone, p > 0.05. The HI + Hypo group also did not differ from sham controls, p > 0.05. The %L1 in lipid rafts was highest in the LPS + HI group, and differed significantly from HI, ps < 0.0001. LPS + HI and LPS + HI + Hypo did not significantly differ, p > 0.05. Together, these results suggest a persistent effect of LPS administration on P7 that was not reversed by hypothermia.
Shown is the mean ± SEM of the % L1CAM in lipid rafts in each of the 5 groups 4 h post-HI (immediately after hypothermia (HT) on both the right (ipsilateral, a) and the left (contralateral, b) sides of the cerebellum. a Right side of cerebellum. There were significant differences between the groups (1 way ANOVA p < 0.0001). There was a significant increase in the %L1CAM in lipid rafts in all treatment groups compared to sham, *ps < 0.001). There was no significant difference between HI and any other treatment group, smallest p < 0.19. b Left side of cerebellum. There was a significant difference between the 5 groups (1 way ANOVA p < 0.0001). The sham control was significantly less than all treatment groups except HI + Hypo (Sham vs HI, *p < 0.01; Sham vs LPS + HI, **p < 0.001; **Sham vs LPS + HI + Hypo, p < 0.001). The %L1CAM in Lipid Rafts in LPS + HI was significantly greater than in that of HI alone (**p < 0.001).
At 72 h following HI, significant group differences were confirmed in both the right, F(4,27) = 7.23, p < 0.0001, and left cerebellum, F(4,27) = 4.59, p < 0.006. The right cerebellum continued to show a significant difference between sham controls and all other treatment groups, ps < 0.007 (Fig. 4). There were no significant differences between the HI-treated groups, all ps > 0.05. Thus, the effect of HI in the right cerebellum was persistent at the 72 h timepoint, and not further modulated by either LPS or hypothermia. The effect of LPS was persistent in the left cerebellum. Both LPS + HI and LPS + HI + Hypo significantly differed from the sham control group, ps < 0.04. HI did not significantly differ from the sham group, p > 0.05. The HI + Hypo group also did not differ from the sham control group, p > 0.05. We did not detect a significant correlation between the injury scores and the percent %L1 in lipid rafts, r = −0.204, p < 0.3. Sample sizes did not permit analysis of changes in distribution across injury scores by experimental group. Across all experimental groups, 61% received an injury score of 2 or higher, indicating an observable infarct at this timepoint.
Shown is the mean ± SEM of the % L1CAM in lipid rafts in each of the 5 treatment groups at 72 h post-HI on both the right (ipsilateral, a) and the left (contralateral, b) sides of the cerebellum. a Right side of cerebellum. There were significant differences between the groups (1 way ANOVA p < 0.0001). There was a significant increase in the %L1CAM in Lipid Rafts in all four groups subjected to HI compared to sham (*ps < 0.007). There was no significant difference between any groups subjected to HI (p > 0.05). b Left side of cerebellum. There was a significant difference between the groups (1 way ANOVA p < 0.006). The sham control was significantly less than both LPS + HI, p < 0.04, and LPS + HI + Hypo, p < 0.003. The %L1CAM in lipid rafts was no longer significantly different between sham and HI, p < 0.08, or sham and HI + Hypo, p > 0.05 (ns). There was no significant difference between any other treatment group (p > 0.05).
Analysis of the right and left sides of the cerebellum at 4 h confirmed that the sides did not differ in sham-operated controls, t(10) = 0.61, p > 0.05 (Table 1). The %L1 in lipid rafts was significantly higher in the right side than the left in all other groups, smallest t(9) = 7.8, p < 0.0001. The same pattern of results persisted at 72 h post-HI. The right and left sides did not differ in the sham-operated group, t(5) = 0.15, p > 0.05. The %L1 in lipid rafts in the right side was higher than that observed in the left in all groups subjected to HI, regardless of LPS or hypothermia, smallest t(6) = 2.3, p < 0.054.
The %L1 in lipid rafts increased between 4 and 72 h in both sides of sham-operated pups, smallest t(15) = 2.58, p < 0.05 (Table 2), most likely due to the increase of L1 in lipid rafts at this time of development.32 The %L1 in lipid rafts from the right cerebellum decreased significantly at 72 h for all treatment groups, although remaining significantly greater than sham (Fig. 4). In contrast, the %L1 in lipid rafts from the left cerebellum significantly increased at 72 h for all treatment groups with the exception of HI + LPS (Table 2). HI + LPS was the only treatment group that didn’t change between time 4 and 72 h.
Discussion
Our study is the first to show that lipid raft composition in the developing cerebellum is changed by HI. HI significantly increased the %L1 in lipid rafts in both the left and right sides of the cerebellum. Administration of LPS increased translocation of L1 to lipid rafts persistently in the left cerebellum, but not significantly above HI alone in the right, ipsilateral to the carotid artery ligation. Similarly, hypothermia did not reverse translocation of L1 to lipid rafts. Lipid rafts are a site of organized aggregation and interaction for various molecules important for brain development and cellular function.31 Organization of proteins within lipid rafts facilitates critical protein interactions, influencing signaling pathways, neurite guidance and outgrowth, and cell modulation by growth factors.32 Lipid rafts also anchor ion channels and synaptic vesicle fusion proteins, influencing membrane properties and neurotransmitter release.45 Translocation of L1 to lipid rafts is tightly developmentally regulated, affecting migration and integration of neurons in the developing brain.46 Disruption of normal L1 traffic through lipid rafts contributes to poor neurite outgrowth of cerebellar granule cells in models of various developmental insults to the premature brain.28,29,30 Here, we present evidence that neonatal HI also disrupts the normal distribution of L1 within lipid rafts in the developing, term equivalent cerebellum.
The observed difference in magnitude in L1 distribution between the right and left cerebellum is not easily explained by changes in blood flow to the cerebellum in this model of neonatal HIE. Though blood flow is dramatically decreased in the cerebral hemisphere ipsilateral to the carotid artery that is ligated, blood flow is acutely increased in both hemispheres of the cerebellum.47,48,49 Compensatory increases in blood flow in the contralateral cerebral hemisphere have been documented, and redistribution of blood flow correlates with better histological outcomes.50 This suggests that increases in blood flow in the contralateral cerebrum, and perhaps also the cerebellum, may be protective.51 Injury in the cerebellum likely evolves as it does in the cerebrum. Histological measures reveal a protracted loss of cerebral volume in both the ipsilateral and contralateral hemisphere detectable in late adolescence and adulthood in response to HI (e.g., refs. 23,52,53). Similarly, cell death was observed in the cerebellar hemisphere ipsilateral to the carotid artery ligation at short post-HI intervals.54 At later timepoints, cell death and metabolic dysregulation were evident in the contralateral cerebellar hemisphere.14,54 The time course of cerebellar response to cerebral injury in the neonate remains unclear, however, and the pattern of cerebellar changes depends on the age at the time of injury.55 The evolution of cerebellar injury over time is likely due to its sensitivity to hypoxia13 as well as its ipsilateral and contralateral connectivity to the cerebrum (e.g., refs. 56,57,58,59).
Studies of postmortem tissue and quantitative imaging analysis in term infants with HIE suggest that cerebellar damage is likely underestimated by qualitative imaging techniques.60,61,62,63 Quantitative analysis of cerebellar growth found a normal trajectory of cerebellar growth, but growth was less in infants with severe HIE, particularly when injury included the basal ganglia.63 Detailed analysis of diffusion tensor imaging (DTI) data also found evidence of damage to white matter tracts passing through the cerebellar peduncle.62 Postmortem histopathology studies in near-term and term infants from whom apparent diffusion coefficient (ADC) data were collected antemortem found that ADC values were abnormal in only severe cases of HIE, while histopathology confirmed morphological abnormalities in Purkinje cells and neuroinflammation in the majority of infants examined.60 Given the role of the cerebellum in motor, cognitive and emotional behavior (reviewed in ref. 64) therapies that can safely augment the efficacy of hypothermia in this late-developing brain region could significantly improve functional outcomes in a range of domains. Whole-body hypothermia is well established as an effective method against mortality and cell death.4,11 Its effectiveness, however, falls drastically as time between HI and initiation of cooling progresses,11 as well as time between HI and birth increases.10,11 It is becoming increasingly clear that the most effective treatment for HIE is a combination of whole-body hypothermia with pharmacological treatments to target specifically vulnerable brain regions.5,65
Systemic LPS administration did not significantly increase L1 redistribution in the right, ischemic cerebellum above HI alone, but did lead to the most persistent elevation of L1 redistribution to lipid rafts in the left cerebellum, irrespective of hypothermia. LPS binding to its target receptor, TLR-4, induces translocation of the receptor to lipid rafts. Reducing recruitment of TLR-4 to lipid rafts decreases inflammation and magnitude of injury after stroke in adult mice.66 Though we cannot conclude that LPS alone increases L1 redistribution to lipid rafts due to our experimental design, it is possible that in the developing brain, inflammation both exacerbates injury and disrupts the normal development of immature cells at the level of the lipid raft. Interventions that both reduce inflammation and injury might enhance the therapeutic efficacy of hypothermia. The polyunsaturated fatty acid (PUFA) docosahexanoic acid (DHA) regulates the formation of lipid rafts and augments the neuroprotective effects of hypothermia.45,67,68,69,70,71,72,73,74 DHA and hypothermia together reduce indices of lipid peroxidation in urine and plasma in a piglet model of HI,75 which may lead to stabilization of lipid rafts. The dependence of lipid raft integrity on DHA and the augmentation of neuroprotection of hypothermia with DHA suggest that lipid raft impairment may be a critical point of intervention in HIE. Interestingly, DHA as well as other PUFAs can reduce inflammation, specifically through their direct influence on TLR-4 ligand binding as well as modulation of downstream signaling pathways.76 Thus, dietary intake of PUFAs might improve the efficacy of hypothermia even in the context of inflammation, as well as promote proper lipid raft function with a low risk of harm in the neonate. Future studies should consider lipid raft integrity as a potential target for intervention.
References
Fatemi, A., Wilson, M. A. & Johnston, M. V. Hypoxic-ischemic encephalopathy in the term infant. Clin. Perinatol. 36, 835–858 (2009).
Graham, E. M., Ruis, K. A., Hartman, A. L., Northington, F. J. & Fox, H. E. A systematic review of the role of intrapartum hypoxia-ischemia in the causation of neonatal encephalopathy. Am. J. Obstet. Gynecol. 199, 587–595 (2008).
Bonifacio, S. L. & Hutson, S. The term newborn: evaluation for hypoxic-ischemic encephalopathy. Clin. Perinatol. 48, 681–695 (2021).
Shankaran, S. et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N. Engl. J. Med. 353, 1574–1584 (2005).
Davidson, J. O., Wassink, G., Van Den Heuij, L. G., Bennet, L. & Gunn, A. J. Therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy - where to from here? Front. Neurol. 6, 198 (2015).
Schreglmann, M., Ground, A., Vollmer, B. & Johnson, M. J. Systematic review: long-term cognitive and behavioural outcomes of neonatal hypoxic–ischaemic encephalopathy in children without cerebral palsy. Acta Paediatr. Int. J. Paediatr. 109, 20–30 (2020).
Askalan, R., Wang, C., Shi, H., Armstrong, E. & Yager, J. Y. The effect of postischemic hypothermia on apoptotic cell death in the neonatal rat brain. Dev. Neurosci. 33, 320–329 (2011).
Wassink, G., Gunn, E. R., Drury, P. P., Bennet, L. & Gunn, A. J. The mechanisms and treatment of asphyxial encephalopathy. Front. Neurosci. 8, 40 (2014).
Wassink, G. et al. A working model for hypothermic neuroprotection. J. Physiol. 596, 5641–5654 (2018).
Chevin, M. et al. Neuroprotective effects of hypothermia in inflammatory-sensitized hypoxic-ischemic encephalopathy. Int. J. Dev. Neurosci. 55, 1–8 (2016).
Colbourne, F., Sutherland, G. & Corbett, D. Postischemic hypothermia. A critical appraisal with implications for clinical treatment. Mol. Neurobiol. 14, 171–201 (1997).
Benitez, S. G., Castro, A. E., Patterson, S. I., Muñoz, E. M. & Seltzer, A. M. Hypoxic preconditioning differentially affects GABAergic and glutamatergic neuronal cells in the injured cerebellum of the neonatal rat. PLoS ONE 9, e102056 (2014).
Biran, V. et al. Cerebellar abnormalities following hypoxia alone compared to hypoxic-ischemic forebrain injury in the developing rat brain. Neurobiol. Dis. 41, 138–146 (2011).
Sanches, E. F., Van De Looij, Y., Toulotte, A., Sizonenko, S. V. & Lei, H. Mild neonatal brain hypoxia-ischemia in very immature rats causes long-term behavioral and cerebellar abnormalities at adulthood. Front. Physiol. 10, 1–12. (2019).
Altman, J. Morphological development of the rat cerebellum and some of its mechanisms. Exp. Brain Res. 47, 8–49 (1982).
Altman, J. & Das, G. D. Autoradiographic and histological studies of postnatal neurogenesis migration and transformation of cells incorporating tritiated thymidine in neonate rats, with special reference to postnatal neurogenesis in some brain regions. J. Comp. Neurol. 126, 337–390. (1966).
Peng, J. H. F., Feng, Y., LeBlanc, M. H., Rhodes, P. G. & Parker, J. C. Apoptosis and necrosis in developing cerebellum and brainstem induced after focal cerebral hypoxic-ischemic injury. Dev. Brain Res. 156, 87–92 (2005).
Volpe, J. J. Cerebellum of the premature infant: rapidly developing, vulnerable, clinically important. J. Child Neurol. 24, 1085–1104 (2009).
Sathyanesan, A. et al. Emerging connections between cerebellar development, behaviour and complex brain disorders. Nat. Rev. Neurosci. 20, 298–313 (2019).
Wang, V. Y. & Zoghbi, H. Y. Genetic regulation of cerebellar development. Nat. Rev. Neurosci. 2, 484–491 (2001).
Levine, S. Anoxic-ischemic encephalopathy in rats. Am. J. Pathol. 36, 1–17 (1960).
Rice, J. E., Vannucci, R. C. & Brierley, J. B. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann. Neurol. 9, 131–141 (1981).
Geddes, R., Vannucci, R. C. & Vannucci, S. J. Delayed cerebral atrophy following moderate hypoxia-ischemia in the immature rat. Dev. Neurosci. 23, 180–185 (2001).
Towfighi, J., Zec, N., Yager, J., Housman, C. & Vannucci, R. C. Temporal evolution of neuropathologic changes in an immature rat model of cerebral hypoxia: a light microscopic study. Acta Neuropathol. 90, 375–386 (1995).
Burden-Gulley, S. M., Pendergast, M. & Lemmon, V. The role of cell adhesion molecule L1 in axonal extension, growth cone motility, and signal transduction. Cell Tissue Res. 290, 415–422 (1997).
Hortsch, M. Structural and functional evolution of the L1 family: are four adhesion molecules better than one? Mol. Cell. Neurosci. 15, 1–10 (2000).
Katidou, M., Vidaki, M., Strigini, M. & Karagogeos, D. The immunoglobulin superfamily of neuronal cell adhesion molecules: lessons from animal models and correlation with human disease. Biotechnol. J. 3, 1564–1580 (2008).
Tang, N. et al. Ethanol causes the redistribution of L1 cell adhesion molecule in lipid rafts. J. Neurochem. 119, 859–867 (2011).
Kitchen, S. T. et al. Bilirubin inhibits lipid raft dependent functions of L1 cell adhesion molecule in rat pup cerebellar granule neurons. Pediatr. Res. 89, 1389–1395 (2021).
Milstone, A. M. et al. Chlorhexidine inhibits L1 cell adhesion molecule-mediated neurite outgrowth in vitro. Pediatr. Res. 75, 8–13 (2014).
Tsui-Pierchala, B. A., Encinas, M., Milbrandt, J. & Johnson, E. M. Lipid rafts in neuronal signaling and function. Trends Neurosci. 25, 412–417 (2002).
Nakai, Y. & Kamiguchi, H. Migration of nerve growth cones requires detergent-resistant membranes in a spatially defined and substrate-dependent manner. J. Cell Biol. 159, 1097–1108 (2002).
Semple, B. D., Blomgren, K., Gimlin, K., Ferriero, D. M. & Noble-Haeusslein, L. J. Brain development in rodents and humans: identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 106–107, 1–16 (2013).
Chow, J. C., Young, D. W., Golenbock, D. T., Christ, W. J. & Gusovsky, F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 274, 10689–10692 (1999).
Rietschel, E. T. et al. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 8, 217–225 (1994).
Olsson, S. & Sundler, R. The role of lipid rafts in LPS-induced signaling in a macrophage cell line. Mol. Immunol. 43, 607–612 (2006).
Miller, Y. I., Navia-Pelaez, J. M., Corr, M. & Yaksh, T. L. Lipid rafts in glial cells: role in neuroinflammation and pain processing. J. Lipid Res. 61, 655–666 (2020).
Eklind, S., Mallard, C., Arvidsson, P. & Hagberg, H. Lipopolysaccharide induces both a primary and a secondary phase of sensitization in the developing rat brain. Pediatr. Res. 58, 112–116 (2005).
Eklind, S. et al. Bacterial endotoxin sensitizes the immature brain to hypoxic-ischaemic injury. Eur. J. Neurosci. 13, 1101–1106 (2001).
Wang, X., Rousset, C. I., Hagberg, H. & Mallard, C. Lipopolysaccharide-induced inflammation and perinatal brain injury. Semin. Fetal Neonatal Med. 11, 343–353 (2006).
Martinello, K. A. et al. Hypothermia is not therapeutic in a neonatal piglet model of inflammation-sensitized hypoxia–ischemia. Pediatr. Res. https://doi.org/10.1038/s41390-021-01584-6 (2021).
Corry, K. A. et al. Evaluating neuroprotective effects of uridine, erythropoietin, and therapeutic hypothermia in a ferret model of inflammation-sensitized hypoxic-ischemic encephalopathy. Int. J. Mol. Sci. 22, 9841 (2021).
Sobesky, J. et al. Crossed cerebellar diaschisis in acute human stroke: a PET study of serial changes and response to supratentorial reperfusion. J. Cereb. Blood Flow. Metab. 25, 1685–1691 (2005).
Patel, S. D. et al. Therapeutic hypothermia and hypoxia-ischemia in the term-equivalent neonatal rat: characterization of a translational preclinical model. Pediatr. Res. 78, 264–271 (2015).
Lingwood, D. & Simons, K. Lipid rafts as a membrane-organizing principle. Science 327, 46–50 (2010).
Maness, P. F. & Schachner, M. Neural recognition molecules of the immunoglobulin superfamily: signaling transducers of axon guidance and neuronal migration. Nat. Neurosci. 10, 19–26 (2006).
Edwards, A. B. et al. Modification to the Rice-Vannucci perinatal hypoxic-ischaemic encephalopathy model in the P7 rat improves the reliability of cerebral infarct development after 48 h. J. Neurosci. Methods 288, 62–71 (2017).
Vannucci, R. C., Lyons, D. T. & Vasta, F. Regional cerebral blood flow during hypoxia-ischemia in immature rats. Stroke 19, 245–250 (1988).
Wood, T. et al. Monitoring of cerebral blood flow during hypoxia-ischemia and resuscitation in the neonatal rat using laser speckle imaging. Physiol. Rep. 4, e12749 (2016).
Ohshima, M., Tsuji, M., Taguchi, A., Kasahara, Y. & Ikeda, T. Cerebral blood flow during reperfusion predicts later brain damage in a mouse and a rat model of neonatal hypoxic–ischemic encephalopathy. Exp. Neurol. 233, 481–489 (2012).
Charriaut-Marlangue, C. et al. Sildenafil mediates blood-flow redistribution and neuroprotection after neonatal hypoxia-ischemia. Stroke 45, 850–856 (2014).
Stone, B. S. et al. Delayed neural network degeneration after neonatal hypoxia-ischemia. Ann. Neurol. 64, 535–546 (2008).
Tang, S. et al. Neuroprotective effects of acetyl-L-carnitine on neonatal hypoxia ischemia-induced brain injury in rats. Dev. Neurosci. 38, 384–396 (2017).
Taylor, D. L., Joashi, U. C., Sarraf, C., Edwards, A. D. & Mehmet, H. Consequential apoptosis in the cerebellum following injury to the developing rat forebrain. Brain Pathol. 16, 195–201 (2006).
Shamoto, H. & Chugani, H. T. Glucose metabolism in the human cerebellum: an analysis of crossed cerebellar diaschisis in children with unilateral cerebral injury. J. Child Neurol. 12, 407–414 (2016).
Onat, F. & Çavdar, S. Cerebellar connections: hypothalamus. Cerebellum 2, 263–269 (2003).
Çavdar, S. et al. Cerebellar connections to the rostral reticular nucleus of the thalamus in the rat. J. Anat. 201, 485–491 (2002).
Watson, T. C. et al. Anatomical and physiological foundations of cerebello-hippocampal interaction. Elife 8, 1–28. (2019).
Xiao, L., Bornmann, C., Hatstatt-Burklé, L. & Scheiffele, P. Regulation of striatal cells and goal-directed behavior by cerebellar outputs. Nat. Commun. 9, 1–14. (2018).
Annink, K. V. et al. Cerebellar injury in term neonates with hypoxic–ischemic encephalopathy is underestimated. Pediatr. Res. 89, 1171–1178 (2021).
Kwan, S. et al. Injury to the cerebellum in term asphyxiated newborns treated with hypothermia. Am. J. Neuroradiol. 36, 1542–1549 (2015).
Lemmon, M. E. et al. Diffusion tensor imaging detects occult cerebellar injury in severe neonatal hypoxic-ischemic encephalopathy. Dev. Neurosci. 39, 207–214 (2017).
Le Strange, E., Saeed, N., Cowan, F. M., Edwards, A. D. & Rutherford, M. A. MR imaging quantification of cerebellar growth following hypoxic-ischemic injury to the neonatal. Brain Am. J. Neuroradiol. 25, 463–468 (2004).
Wang, S. S. H., Kloth, A. D. & Badura, A. The cerebellum, sensitive periods, and autism. Neuron 83, 518–532 (2014).
Zhou, K. Q., Davidson, J. O., Bennet, L. & Gunn, A. J. Combination treatments with therapeutic hypothermia for hypoxic-ischemic neuroprotection. Dev. Med. Child Neurol. 62, 1131–1137 (2020).
Xue, J. et al. Sphingomyelin synthase 2 inhibition ameliorates cerebral ischemic reperfusion injury through reducing the recruitment of toll-like receptor 4 to lipid rafts. J. Am. Heart Assoc. 8, 1–14. (2019).
Berman, D. R. et al. Docosahexaenoic acid augments hypothermic neuroprotection in a neonatal rat asphyxia model. Neonatology 104, 71–78 (2013).
Wassall, S. R. et al. Docosahexaenoic acid regulates the formation of lipid rafts: A unified view from experiment and simulation. Biochim. Biophys. Acta Biomembr. 1860, 1985–1993 (2018).
Kinnun, J. J., Bittman, R., Shaikh, S. R. & Wassall, S. R. DHA modifies the size and composition of raftlike domains: a solid-state 2H NMR study. Biophys. J. 114, 380–391 (2018).
Shaikh, S. R., Kinnun, J. J., Leng, X., Williams, J. A. & Wassall, S. R. How polyunsaturated fatty acids modify molecular organization in membranes: insight from NMR studies of model systems. Biochim. Biophys. Acta Biomembr. 1848, 211–219 (2015).
Shaikh, S. R., Rockett, B. D., Salameh, M. & Carraway, K. Docosahexaenoic acid modifies the clustering and size of lipid rafts and the lateral organization and surface expression of MHC class I of EL4 cells. J. Nutr. 139, 1632–1639 (2009).
Hou, T. Y. et al. N-3 polyunsaturated fatty acids suppress CD4+ T cell proliferation by altering phosphatidylinositol-(4,5)-bisphosphate [PI(4,5)P2] organization. Biochim. Biophys. Acta Biomembr. 1858, 85–96 (2016).
Levental, I. & Veatch, S. L. The continuing mystery of lipid rafts. J. Mol. Biol. 428, 4749–4764 (2016).
Schley, P. D., Brindley, D. N. & Field, C. J. (n-3) PUFA alter raft lipid composition and decrease epidermal growth factor receptor levels in lipid rafts of human breast cancer cells. J. Nutr. 137, 548–553 (2007).
Huun, M. U. et al. DHA reduces oxidative stress following hypoxia-ischemia in newborn piglets: a study of lipid peroxidation products in urine and plasma. J. Perinat. Med. 46, 209–217 (2018).
Jalili, M. & Hekmatdoost, A. Dietary ω-3 fatty acids and their influence on inflammation via Toll-like receptor pathways. Nutrition 85, 111070 (2021).
Funding
This study was funded by the NIH/NICHD P01 HD085928.
Author information
Authors and Affiliations
Contributions
J.W.: Substantial contributions to gathering and interpretation of data, drafting and revising article critically for important intellectual content, and final approval of the version to be published. N.C.R.: Substantial contribution to drafting the article or revising it critically for important intellectual content. M.H.: Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data. N.T.: Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data. C.F.B.: Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data, drafting the article or revising it critically for important intellectual content; and final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Disclaimer
This material is original and has not been previously published nor has it been submitted for publication elsewhere while under consideration.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Waddell, J., Rickman, N.C., He, M. et al. Neonatal hypoxia ischemia redistributes L1 cell adhesion molecule into rat cerebellar lipid rafts. Pediatr Res 92, 1325–1331 (2022). https://doi.org/10.1038/s41390-022-01974-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-01974-4