Abstract
Background
Severe retinopathy of prematurity (ROP) is associated with adverse outcomes. Relationships between milder ROP and outcomes have not been defined. We hypothesized that children with ROP stage ≤3 who did not receive ophthalmologic intervention would have worse motor, cognitive, and language skills and more vision abnormalities than children without ROP.
Methods
This was a secondary analysis of a randomized trial evaluating the effects of myo-inositol on ROP in the NICHD Neonatal Research Network. Primary outcomes were Bayley Scales of Infant Development composite scores; secondary outcomes included behavioral difficulties and ophthalmologic measures. Outcomes were compared using adjusted linear or modified Poisson models.
Results
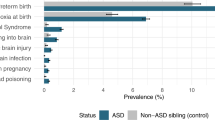
Of 506 children, 173 (34%) had no ROP, 262 (52%) had ROP stage ≤3 without intervention, and 71 (14%) had ROP with intervention. There was no difference in motor, cognitive, or language scores between children with ROP stage ≤3 without intervention and children without ROP. Children with ROP stage ≤3 without intervention had a higher rate of strabismus compared to children without ROP (p = 0.040).
Conclusion
Children with ROP stage ≤3 without intervention did not have adverse neurodevelopmental outcomes at 2 years’ corrected age compared to children without ROP but did have an increased incidence of strabismus.
Impact
-
This study addresses a gap in the literature regarding the relationship between milder forms of retinopathy of prematurity (ROP) that regress without intervention and neurodevelopment and vision outcomes.
-
Children with a history of ROP stage ≤3 without intervention have similar neurodevelopmental outcomes at 2 years’ corrected age as children born extremely preterm without a history of ROP and better outcomes than children with a history of ROP with ophthalmologic intervention.
-
Counseling about likely neurodevelopment and vision outcomes for children born extremely preterm with a history of ROP may be tailored based on the severity of ROP.
Clinical trial registration
ClinicalTrials.gov ID: Inositol to Reduce Retinopathy of Prematurity Trial: NCT01954082.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
Data reported in this paper may be requested through a data use agreement. Further details are available at https://neonatal.rti.org/index.cfm?fuseaction=DataRequest.Home.
References
Hartnett, M. E. Pathophysiology and mechanisms of severe retinopathy of prematurity. Ophthalmology 122, 200–210 (2015).
Allred, E. N. et al. Retinopathy of prematurity and brain damage in the very preterm newborn. J. Aapos. 18, 241–247 (2014).
Holmström, G. E. et al. Ophthalmologic outcome at 30 months’ corrected age of a prospective Swedish cohort of children born before 27 weeks of gestation: the extremely preterm infants in Sweden study. JAMA Ophthalmol. 132, 182–189 (2014).
Hellgren, K. M. et al. Ophthalmologic outcome of extremely preterm infants at 6.5 years of age: extremely preterm infants in Sweden Study (EXPRESS). JAMA Ophthalmol. 134, 555–562 (2016).
Schmidt, B., Davis, P. G., Asztalos, E. V., Solimano, A. & Roberts, R. S. Association between severe retinopathy of prematurity and nonvisual disabilities at age 5 years. JAMA 311, 523–525 (2014).
Glass, T. J. A. et al. Severe retinopathy of prematurity predicts delayed white matter maturation and poorer neurodevelopment. Arch. Dis. Child. Fetal Neonatal Ed. 102, F532–F537 (2017).
Natarajan, G. et al. Neurodevelopmental outcomes of preterm infants with retinopathy of prematurity by treatment. Pediatrics 144, e20183537 (2019).
Phelps, D. L. et al. Effects of myo-inositol on type 1 retinopathy of prematurity among preterm infants <28 weeks’ gestational age: a randomized clinical trial. JAMA 320, 1649–1658 (2018).
Albers, C. A. & Grieve, A. J. Bayley Scales of Infant and Toddler Development, 3rd edition. J. Psychoeduc. Assess. 25, 180–190 (2007).
Achenbach, T. M., & Rescorla, L. A. Manual for the ASEBA School-age Forms & Profiles. (University of Vermont Research Center for Children, Youth, & Families, Burlington, VT, 2001).
Palisano, R. et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev. Med. Child Neurol. 39, 214–223 (1997).
Alexander, G. R., Himes, J. H., Kaufman, R. B., Mor, J. & Kogan, M. A United States national reference for fetal growth. Obstet. Gynecol. 87, 163–168 (1996).
Papile, L. A., Burstein, J., Burstein, R. & Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J. Pediatr. 92, 529–534 (1978).
de Verdier, K., Ulla, E., Löfgren, S. & Fernell, E. Children with blindness – major causes, developmental outcomes and implications for habilitation and educational support: a two-decade, Swedish population-based study. Acta Ophthalmol. 96, 295–300 (2018).
Chau, V. et al. Abnormal brain maturation in preterm neonates associated with adverse developmental outcomes. Neurology 81, 2082–2089 (2013).
Sveinsdóttir, K. et al. Relation of retinopathy of prematurity to brain volumes at term equivalent age and developmental outcome at 2 years of corrected age in very preterm infants. Neonatology 114, 46–52 (2018).
Sampath, V., Bowen, J. & Gibson, F. Risk factors for adverse neurodevelopment in extremely low birth weight infants with normal neonatal cranial ultrasound. J. Perinatol. 25, 210–215 (2005).
Msall, M. E. et al. Severity of neonatal retinopathy of prematurity is predictive of neurodevelopmental functional outcome at age 5.5 years. Pediatrics 106, 998–1005 (2000).
Molloy, C. S., Anderson, P. J., Anderson, V. A. & Doyle, L. W. The long-term outcome of extremely preterm (<28 weeks’ gestational age) infants with and without severe retinopathy of prematurity. J. Neuropsychol. 10, 276–294 (2016).
Costa, D. S. et al. Executive function and academic outcomes in children who were extremely preterm. Pediatrics 140, e20170257 (2017).
Joseph, R. M. et al. Neurocognitive and academic outcomes at age 10 years of extremely preterm newborns. Pediatrics 137, e20154343 (2016).
Palmer, E. A. et al. 15-year outcomes following threshold retinopathy of prematurity: final results from the multicenter trial of cryotherapy for retinopathy of prematurity. Arch. Ophthalmol. 123, 311–318 (2005).
Acknowledgements
The National Institutes of Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) through the Neonatal Research Network, and the National Eye Institute (NEI) provided grant support for the Inositol Trial. While NICHD staff had input into the study design, conduct, analysis, and manuscript drafting, the comments and views of the authors do not necessarily represent the views of NICHD, NEI, the National Institutes of Health, the Department of Health and Human Services, or the U.S. Government. Participating NRN sites collected data and transmitted it to RTI International, the data coordinating center (DCC) for the network, which stored, managed, and analyzed the data for this study. On behalf of the NRN, RTI International had full access to all of the data in the study and, with the NRN Center Principal Investigators, takes responsibility for the integrity of the data and accuracy of the data analysis. We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study. The following investigators, in addition to those listed as authors, participated in this study: NRN Steering Committee Chair: Richard A. Polin, MD, Division of Neonatology, College of Physicians and Surgeons, Columbia University (2011-present). Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island (UG1 HD27904): Abbot R. Laptook, MD; Martin Keszler, MD; William Oh, MD; Elisabeth C. McGowan, MD; John P. Donahue, MD, PhD; Angelita M. Hensman, PhD, RNC-NIC; Barbara Alksninis, PNP; Mary Lenore Keszler, MD; Andrea M. Knoll; Theresa M. Leach, MEd; Emilee Little, RN, BSN; Elisabeth C. McGowan, MD; Michael R. Muller, PharmD; Elisa Vieira, RN, BSN; Victoria E. Watson, MS, CAS. Case Western Reserve University, Rainbow Babies & Children’s Hospital (UG1 HD21364); Michele C. Walsh, MD, MS; Anna Maria Hibbs, MD, MSCE; Nancy S. Newman, BA, RN; Deanne E. Wilson-Costello, MD; Faruk H. Orge, MD; Michael Banchy, RPH; Monika Bhola, MD; Jeffrey L. Blumer, MD; Allison H. Payne, MD, MS; Bonnie S. Siner, RN; Elizabeth Ross, MS; Eileen K. Stork, MD; H. Gerry Taylor, PhD; Gulgun Yalcinkaya, MD; Arlene Zadell, RN. Children’s Mercy Hospital, University of Missouri Kansas City School of Medicine (UG1 HD68284): William E. Truog, MD; Howard W. Kilbride, MD; Denise Hug, MD; Eugenia K. Pallotto, MD, MSCE; Prabhu S. Parimi, MD; Cheri Gauldin, RN, BSN, CCRC; Lisa Gaetano, MSN, RN; Anne M. Holmes, RN, MSN, MBA-HCM, CCRC; Kathy Johnson RN, CCRC; Allison Knutson, BSN, RNC-NIC. Cincinnati Children’s Hospital Medical Center, University of Cincinnati Medical Center, and Good Samaritan Hospital (UG1 HD27853, UL1 TR77): Brenda B. Poindexter, MD, MS; Kurt Schibler, MD; Cathy Grisby, BSN, CCRC; Stephanie Merhar, MD, MS; Michael B. Yang, MD; Patricia Cobb, MS; Teresa L. Gratton, PA; Kristin Kirker, CRC; Stacey Tepe, BS; Sandra Wuertz, RN-BSN, CCRP, CLC; Kimberly Yolton, PhD. Duke University School of Medicine, University Hospital, University of North Carolina, Duke Regional Hospital, and WakeMed Health and Hospitals (UG1 HD40492, UL1 TR1117); C. Michael Cotten, MD, MHS; Ronald N. Goldberg, MD; Joanne Finkle, RN, JD; Kimberley A. Fisher, PhD, FNP-BC, IBCLC; Ricki F. Goldstein, MD; William F. Malcolm, MD; David K. Wallace, MD, MPH; Patricia L. Ashley, MD, PHD; Deesha Mago-Shah, MD; Chi Dang-Hornik, PharmD, BCPS; Sharon F. Freedman, MD; Kathryn E. Gustafson, PhD; Mary Miller-Bell, PharmD, RPh; Sasapin Grace Prakalapakorn, MD, MPH; Matthew M. Laughon, MD, MPH; Carl L. Bose, MD; Janice Bernhardt, MS, RN; Cindy Clark, RN; Diane D. Warner, MD, MPH; Michael T. O’Shea, MD, MPH; Janice Wereszczak, CPNP-AC/PC; Jennifer Talbert, MS, RN; Stephen D. Kicklighter, MD; Sofia Aliaga, MD, MPH; Jeffery Board, MD; Kevin Gertsch, MD; Jerry Magolan, MD; Linda Manor, RPh; Jan Niklas Ulrich, MD; Ginger Rhodes-Ryan, ARNP, MSN, NNP-BC; Donna White, BSN, RN-BC, BSN; Alexandra Bentley, MD; Laura Edwards, MD. Emory University, Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory University Hospital Midtown (UG1 HD27851, UL1 TR454): David P. Carlton, MD; Barbara J. Stoll, MD; Ellen C. Hale, RN, BS, CCRC; Yvonne Loggins, RN; Amy K. Hutchinson, MD; Diane I. Bottcher, RN, MSN; Sheena L. Carter, PhD; Colleen Mackie, BS, RT; Maureen Mulligan LaRossa, RN; Lynn C. Comerford, NNP; Gloria Smike, PNP, MSN; Salathiel Kendrick-Allwood, MD; Angela Leon-Hernandez, MD. Eunice Kennedy Shriver National Institute of Child Health and Human Development: Rosemary D. Higgins, MD; Stephanie Wilson Archer, MA. Indiana University, Riley Hospital for Children and Methodist Hospital at Indiana University Health (UG1 HD27856): Gregory M. Sokol, MD; Brenda B. Poindexter, MD, MS; Kathryn M. Haider, MD; Susan Gunn, NNP, CCRC; Dianne E. Herron, RN, CCRC; Abbey C. Hines, PsyD; Elizabeth Hynes, RNC-NIC; Lu-Ann Papile, MD; Lucy Smiley, CCRC. McGovern Medical School at The University of Texas Health Science Center at Houston and Children’s Memorial Hermann Hospital (UG1 HD87229): Jon E. Tyson, MD, MPH; Kathleen A. Kennedy, MD, MPH; Amir M. Khan, MD; Helen Mintz-Hittner, MD; Elizabeth Allain, MS; Julie Arldt-McAlister, MSN, APRN; Shanti Brown, RCPhT; Allison G. Dempsey, PhD; Andrea F. Duncan, MD, MSClinRes; Elizabeth Eason, MD; Farida El-Ali, RPH; Carmen Garcia, RN, BSN; Kartik Kumar, MD; Janice John, CPNP; Patrick M. Jones, MD, MA; M. Layne Lillie, RN, BSN; Karen Martin, RN; Sara C. Martin, RN; Georgia E. McDavid, RN; Shannon McKee, EdS; Hatice Ozsoy, PhD, RPh; Shawna Rodgers, RN; Daniel Sperry, RN; Emily K. Stephens, RN, BSN; Vu Ta, PharmD; Christine Wong, PharmD; Sharon L. Wright, MT (ASCP). Nationwide Children’s Hospital and The Ohio State University Wexner Medical Center (UG1 HD68278): Pablo J. Sánchez, MD; Leif D. Nelin, MD; Sudarshan R. Jadcherla, MD; Keith O. Yeates, PhD; Don L. Bremer, MD; Amanda E. Graf, MD; Patricia Luzader, RN; Christine A. Fortney, PhD, RN; Gail E. Besner; Nehal A. Parikh, MD; David L. Rogers, MD; Richard P. Golden, MD; Catherine Olson Jordan, MD. RTI International (U10 HD36790): Abhik Das, PhD; Tracy L. Nolen, DrPH; Dennis Wallace, PhD; Marie G. Gantz, PhD; Carla M. Bann, PhD; Jeanette O’Donnell Auman, BS; Margaret M. Crawford, BS, CCRP; Jenna Gabrio, MPH, CCRP; Carolyn M. Petrie Huitema, MS, CCRP; James W. Pickett II, BS; Annie M. VonLehmden, BS; Kristin M. Zaterka-Baxter, RN, BSN. Stanford University and Lucile Packard Children’s Hospital (UG1 HD27880, UL1 TR93): Krisa P. Van Meurs, MD; David K. Stevenson, MD; M. Bethany Ball, BS, CCRC; Michael Gaynon, MD; Steven Chinn, PharmD; Melinda S. Proud, RCP; Barbara Bentley, PsychD, MSEd; Maria Elena DeAnda, PhD; Anne M. DeBattista, RN, PNP, PhD; Beth Earhart, PhD; Lynne C. Huffman, MD; Casey E. Krueger, PhD; Ryan Lucash, PhD; Hali E. Weiss, MD. University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (UG1 HD34216): Waldemar A. Carlo, MD; Namasivayam Ambalavanan, MD; Monica V. Collins, RN, BSN, MaEd; Shirley S. Cosby, RN, BSN; Myriam Peralta-Carcelen, MD, MPH; Martin S. Cogen, MD; Rebecca J. Quinn, PharmD; Brenda Reed Denson, PharmD; Ann Marie Arciniegas-Bernal, MD; Fred J. Biasini, PhD; Kristen C. Johnston, MSN, CRNP; Cryshelle S. Patterson, PhD; Vivien A. Phillips, RN, BSN; Sally Whitley, MA, OTR-L FAOTA. University of California – Los Angeles, Mattel Children’s Hospital, Santa Monica Hospital, Los Robles Hospital and Medical Center, and Olive View Medical Center (UG1 HD68270): Uday Devaskar, MD; Meena Garg, MD; Teresa Chanlaw, MPH; Rachel Geller, RN, BSN; Isabell B. Purdy, PhD, CPNP; Irena Tsui, MD. University of Iowa (UG1 HD53109, UL1 TR442): Karen J. Johnson, RN, BSN; Richard J. Olson, MD; Jacky R. Walker, RN; Claire A. Goeke, RN; Kristine M. Johnson, BSPharm, RPh; Angela Merriss, BA, CPhT; Joanna L. Nohr, PharmD, BCPS; Susannah Q. Longmuir, MD; Arlene V. Drack, MD; Diane L. Eastman, RN, CPNP, MA; Scott A. Larson, MD; Kevin R. Gertsch, MD; Vikki P. Bell. University of New Mexico Health Sciences Center (UG1 HD53089, UL1 TR41): Robin K. Ohls, MD; Conra Backstrom Lacy, RN; Sandra Sundquist Beauman, MSN, RNC; Janell Fuller, MD; Timothy W. Winter, DO; Tara Dupont, MD; Mary Ruffaner Hanson, RN, BSN; Carol H, Hartenberger, MPH, RN; Elizabeth Kuan, RN, BSN; Susan J. Kunkel, PharmD; Jean Lowe, PhD; Nancy A. Morgan, RPh, MBA. University of Oulu, and Oulu University Hospital, Oulu, Finland: Mikko K. Hallman, MD. University of Pennsylvania, Hospital of the University of Pennsylvania, Pennsylvania Hospital, and Children’s Hospital of Philadelphia (UG1 HD68244): Barbara Schmidt, MD, MSc; Haresh Kirpalani, MB, MSc; Graham Quinn, MD, MSCE; Soraya Abbasi, MD; Aasma S. Chaudhary, BS, RRT; Toni Mancini, RN, BSN, CCRC; William V. Anninger, MD; Judy C. Bernbaum, MD; Gil Binenbaum, MD, MSCE; Noah Cook, MD; Stefanie L. Davidson, MD; Marsha Gerdes, PhD; Hallam Hurt, MD; Monte D. Mills, MD; Mina Ricciardelli, PharmD; Kenneth Rockwell, Jr., PharmD, MS; Jonathan Snyder, RN, BSN; Sze Man Yau, RPh. University of Rochester Medical Center, Golisano Children’s Hospital, and the University of Buffalo Women’s and Children’s Hospital of Buffalo (UG1 HD68263, UL1 TR42): Dale L. Phelps, MD; Carl D’Angio, MD; Satyan Lakshminrusimha, MD; Carol A. Cole, RPh; Anne Marie Reynolds, MD, MPH; Gary J. Myers, MD; Mina Chung, MD (deceased); Stephen A. Bean, PharmD; Melissa F. Carmen, MD; Patricia R. Chess, MD; Rosemary Jensen; Rajeev S. Ramchandran, MD; Ann Marie Scorsone, MS; Ann Marie Turner, PharmD; Ashley Williams, MS, Ed; Michael G. Sacilowski, MAT; Holly Wadkins, MA; Julianne Hunn; Aimee Horan, LPN; Melissa Bowman, RN, NP; Michele Hartley-McAndrew, MD; William Zorn, PhD; Osman Farooq, MD; Kelley Yost, PhD; Joan Merzbach, LMSW; Cait Fallone, MA; Kyle Binion, BS; Constance Orme; Premini Sabaratnam, MPH. University of Texas Southwestern Medical Center, Parkland Health & Hospital System, and Children’s Medical Center Dallas (UG1 HD40689): Myra H. Wyckoff, MD; Luc P. Brion, MD; Diana M. Vasil, RNC-NIC; Roy J. Heyne, MD; Yu-Guang He, MD; Sally S. Adams, MS, RN, CPNP; Christine Cha, PharmD; Juana Cisneros, RN; Maria M. De Leon, BSN, RN; Frances Eubanks, BSN, RN; Lynda Godowic, PharmD, RPh; Laura Grau, RN; Alicia Guzman; Elizabeth Heyne, PsyD, PA-C; Lizette E. Lee, RN; Helen C. Lira, PharmD; Azadeh Mozaffari, PharmD, RPh; Lara Pavageau, MD; Catherine Twell Boatman, MS, CIMI; Reshma Wright, RPh. Wayne State University, Hutzel Women’s Hospital and Children’s Hospital of Michigan (UG1 HD21385): Seetha Shankaran, MD; William R. Lucas, Jr., MD; Beena G. Sood, MD, MS; Rebecca Bara, RN, BSN; Prashant Agarwal, MD; Monika Bajaj, MD; Sanjay Chawla, MD; Kirsten Childs, RN, BSN; Melissa February, MD; Laura A. Goldston, MA; Mary E. Johnson, RN, BSN; Mirjana Lulic-Botica, RPh; Bogdan Panaitescu, MD; Eunice Woldt, RN, MSN.
Funding
The study was funded by the National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (U10 HD36790, UG1 HD27904, UG1 HD21364, UG1 HD68284, UG1 HD27853, UG1 HD40492, UG1 HD27851, UG1 HD27856, UG1 HD87229, UG1 HD68278, UG1 HD27880, UG1 HD34216, UG1 HD68270, UG1 HD53109, UG1 HD53089, UG1 HD68244, UG1 HD68263, UG1 HD40689, UG1 HD21385), the National Eye Institute (via co-funding to NICHD), and the National Center for Advancing Translational Sciences (UL1 TR41, UL1 TR42, UL1 TR77, UL1 TR93, UL1 TR442, UL1 TR454, UL1 TR1117).
Author information
Authors and Affiliations
Consortia
Contributions
Substantial contributions to conception and design: all authors. Acquisition of data: J. E.B., E.F.B., S.B.D., I.S.A.-C., J.R.L., G.N., M.H.W., B.R.V., T.T.C., H.M.H., K.L.W., and S.R.H. Analysis and interpretation of data: J.E.B., E.F.B., S.C.H., and E.G.C. Drafting the article: J.E.B. and E.F.B. Revising the article critically: all authors. Final approval of the version submitted for publication: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors have no potential or perceived conflicts of interest to disclose relevant to this manuscript. None of the authors report any commercial, proprietary, or financial interest in any of the products described in this article. NICHD is the sponsor of the study and holds the investigational new drug (IND) application. Abbott Nutrition Division, Abbott Laboratories, Columbus, OH, provided the myo-inositol product. They had no role in the design of the trial; the analyses, interpretation, or writing of the manuscript; or the decision to submit the manuscript for publication. They provided on-site monitoring to assist in quality assurance of the data collection.
Consent to participate
This study was a secondary analysis of the “Inositol to Reduce Retinopathy of Prematurity” trial (NCT01954082); informed consent was required for participation in this trial.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A list of contributors and their affiliations appears in the Acknowledgements section.
Supplementary information
Rights and permissions
About this article
Cite this article
Brumbaugh, J.E., Bell, E.F., Hirsch, S.C. et al. Relationships between retinopathy of prematurity without ophthalmologic intervention and neurodevelopment and vision at 2 years. Pediatr Res 94, 1720–1730 (2023). https://doi.org/10.1038/s41390-021-01778-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01778-y
This article is cited by
-
Improving neurodevelopmental trajectories after retinopathy of prematurity: challenges and opportunities
Pediatric Research (2023)