Abstract
Background
Preterm born children are at high risk for adverse motor neurodevelopment. The aim of this study was to establish the relationship between motor outcome and advanced magnetic resonance imaging (MRI) and electroencephalography (EEG) measures.
Methods
In a prospective cohort study of 64 very preterm born children, the motor outcome was assessed at 9.83 (SD 0.70) years. Volumetric MRI, diffusion tensor imaging (DTI), and EEG were acquired at 10.85 (SD 0.49) years. We investigated associations between motor outcome and brain volumes (white matter, deep gray matter, cerebellum, and ventricles), white matter integrity (fractional anisotropy and mean, axial and radial diffusivity), and brain activity (upper alpha (A2) functional connectivity and relative A2 power). The independence of associations with motor outcome was investigated with a final model. For each technique, the measure with the strongest association was selected to avoid multicollinearity.
Results
Ventricular volume, radial diffusivity, mean diffusivity, relative A2 power, and A2 functional connectivity were significantly correlated to motor outcome. The final model showed that ventricular volume and relative A2 power were independently associated with motor outcome (B = −9.42 × 10−5, p = 0.027 and B = 28.9, p = 0.007, respectively).
Conclusions
This study suggests that a lasting interplay exists between brain structure and function that might underlie motor outcome at school age.
Impact
-
This is the first study that investigates the relationships between motor outcome and brain volumes, DTI, and brain function in preterm born children at school age.
-
Ventricular volume and relative upper alpha power on EEG have an independent relation with motor outcome in preterm born children at school age.
-
This suggests that there is a lasting interplay between structure and function that underlies adverse motor outcome.
Similar content being viewed by others
Introduction
In the past decades, rapid advances have been booked in the care of newborn babies, resulting in increased survival rates of preterm born infants.1 This has led to a considerably greater number of very preterm infants, i.e., infants born <32 weeks of gestational age (GA), who reach childhood and adolescence. Previous research has demonstrated that very preterm infants are at high risk for long-term neurodevelopmental impairments, including motor deficits.2,3,4
There is considerable heterogeneity in neurodevelopmental outcomes in preterm born children. Several neuromodalities, such as volumetric magnetic resonance imaging (MRI), diffusion tensor imaging (DTI), and electroencephalography (EEG), make it possible to monitor altered brain development.5,6 For example, MRI studies that investigated brain volumes in very preterm born children revealed substantial volume reductions in several brain areas, including white matter, deep gray nuclei, and the cerebellum.7,8 Changes in gray matter and white matter volumes seem to remain present up to early adult life.9
Structural changes in the brains of very preterm born children are possibly related to changes in brain function. Brain activity can be studied by EEG.10 Two commonly used EEG analysis techniques are1 power spectrum analysis, which provides insight into the oscillatory behavior of the brain, and2 functional connectivity analysis, which measures the synchronization of oscillations at two spatially remote locations. A previous study in our cohort revealed that motor outcome was positively correlated to brain activity, more specifically relative upper alpha (A2) power and A2 functional connectivity.11 In addition, Twilhaar et al. showed that the power of alpha oscillations was related to long-term motor outcome in very preterm born adolescents.12 However, the mechanisms that link adverse motor outcome to brain function in the preterm born population remain to be elucidated.
DTI is used to explore changes in microstructural organization and integrity of the white matter.13,14 Commonly used parameters of DTI are fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AxD), and radial diffusivity (RD), reflecting the overall directionality of water flow, the degree of isotropic diffusion, the degree of isotropic diffusion along the axon, and the degree of isotropic diffusion perpendicular to the axon, respectively.15 Diffuse white matter abnormalities have been observed in the very preterm born population from the neonatal period through adulthood and strong associations have been established with motor functioning.16,17,18,19,20,21,22,23 Nowadays, it is thought that diffuse white matter abnormalities play a key role in adverse motor outcome in the preterm born population.24
The majority of studies in the very preterm born population has investigated preterm birth-related brain differences by using one modality. However, earlier studies have noted the importance of combining functional with structural imaging data in a wide age and pathology range.25 To date, subject-specific prognostication and effective intervention strategies for the preterm born population are limited. This is due to limited scientific understanding of the mechanisms underlying adverse long-term outcomes. Combining data derived from multiple neuromodalities has the potential to provide novel insights into pathophysiological mechanisms underlying adverse long-term outcome after very preterm birth.
The aim of this study was to assess the relationship between motor outcome and measures from volumetric MRI, DTI, and EEG 9–10 years after very preterm birth, and—more importantly—to determine if the combination of data derived from multiple neuromodalities would aid in explaining the observed variance in motor outcome in very preterm born children. We hypothesized that there would be a relationship between motor outcome and brain volumes and white matter integrity, as shown in previous literature. In addition, we hypothesized that the combination of measures derived from multiple neuromodalities would increase the proportion of explained variance in motor outcome in very preterm born children.
Methods
Participants and procedures
Participants were part of a prospective cohort of 113 very preterm infants (GA < 32 weeks) born between May 2006 and October 2007. They were previously recruited for a study on neonatal brain imaging and short-term outcome and underwent neonatal MRI and neurodevelopmental follow-up at 2 years corrected age.26,27 At 9–10 years of age, all children and their families were invited for neurodevelopmental follow-up as part of routine clinical care. From the original cohort, 85 very preterm born children were available and underwent standardized neurodevelopmental testing, which included pediatric and neurologic examination, neuromotor evaluation, and neuropsychologic testing. During the follow-up visit, parents and children were informed about the MRI and EEG study, and informed consent for participation was obtained. Exclusion criteria were congenital or acquired abnormalities of the central nervous system or any medical condition that paused a safety issue or made proper preparation for the MRI examination not possible, such as severe motor disability or visual impairment.
The study was conducted in accordance with the Declaration of Helsinki and Medical Research Involving Human Subjects Act (WMO) and was approved by the ethical committee of the LUMC (P17.087; ABR NL59355.058.17). Written consent was obtained from both parents, and all children received age-specific information about the study, including a mock MRI session. When there was resistance, the MRI or EEG was discontinued and only the already available data were included.
Perinatal characteristics
For each child, perinatal data were collected from patient files. These included GA, birth weight (BW), small for gestational age (SGA), sex, postnatal dexamethasone treatment, bronchopulmonary dysplasia (BPD), culture-proven sepsis, necrotizing enterocolitis (NEC), high-grade intraventricular hemorrhage (IVH), and MRI score at term-equivalent age (TEA). BPD was categorized as none or mild/moderate/severe BPD;28 NEC was defined as stage 2 or above.29 SGA as BW below the 10th percentile;30 high-grade IVH included IVH grade III and/or IVH with periventricular hemorrhagic infarction,31 and sepsis as a positive blood culture. The global brain abnormality score on MRI at TEA was based on the Kidokoro scoring system.32
Developmental testing
The Movement Assessment Battery for Children, second Dutch edition (M-ABC-II-NL) was used to assess motor function. The mean age during motor evaluation was 9.83 years (SD 0.70 years). Age band two, i.e., the band normalized for ages 7–11 years, was used and assessment was conducted by an experienced pediatric physiotherapist. The M-ABC-II-NL includes eight tasks, covering three component scores for manual dexterity, ball skills (aiming and catching), and balance (static and dynamic). The M-ABC-II-NL results in a scaled score (normal: any scaled score above 7, i.e., >15th percentile; mildly impaired: scaled score of 6 or 7, i.e., 6–15th percentile; severely impaired: scaled score up to and including 5, i.e., < 5th percentile).
MRI acquisition and processing
MRI examinations were planned separately from follow-up visits. All images were analyzed for quality by a neuroscientist (A.A.v.d.B.-H.) and scored by an experienced neuroradiologist (F.T.W.-d.B.). The assessors were blinded to clinical characteristics and neurodevelopmental outcome of each child.
Structural MRIs were obtained with a 3 Tesla MRI-scanner (Philips, The Netherlands). Anatomical 3D T1-weighted images were acquired in the transverse plane (repetition time (TR) = 9.8 ms, echo time (TE) = 4.6 ms, field of view (FOV) = 177 × 224 cm2, matrix size = 152 × 192, number of slices = 130, slice thickness = 1.2 mm, flip angle = 8°). Volumes of white matter and cerebellum, and bilateral volumes of the thalamus, caudate nucleus, putamen, pallidum, and lateral and inferior ventricles were estimated for each child by using the FreeSurfer Image Analysis Software v.6.0.0. In short, this semi-automated reconstruction pipeline includes motion correction, exclusion of non-brain tissue, affine registration, skull stripping, and gray or white matter voxel classification and intensity normalization.33,34 Parcellation of cortical and subcortical regions was subsequently performed based on probabilistic atlases and subject-specific measures. We defined deep gray matter as the sum of the standardized residuals of the bilateral volumes of the thalamus, caudate nucleus, putamen, and pallidum. Original volumes and volumes as a percentage of the total intracranial volume were investigated. Per subject, post-processed data were visually inspected and analyzed by an experienced neuroscientist (A.A.v.d.B.-H.), blinded to clinical characteristics and neurodevelopmental outcome of each child, and scored on a four-point scale, with one indicating high quality and four indicating low quality. Scans with low quality (score 4) were excluded from the analyses.
DTI acquisition and processing
A single-shot echo-planar DTI sequence was applied with directional diffusion weighting in 32 gradient directions for 2080 volumes per subject (b value = 1000 s/mm2, TR = 15000 ms, TE = 56 ms, FOV = 200 × 240 cm2, matrix size = 100 × 117, number of slices = 65, slice thickness = 2 mm, flip angle = 90°, reconstruction voxel = 2.00 × 2.00 × 2.00 mm2, halfscan factor = 0.61). The 36 directions were uniformly distributed and acquired over the full sphere. One image without diffusion weighting (b value = 0 s/mm2) was acquired for each slice. DTI acquisition time was 4–5 min.
DTI analysis was performed using the FMRIB’s Diffusion Toolbox) and Tract-Based Spatial Statistics (TBSS) package from the FSL Software. Motion and eddy current corrections were performed, and each image was aligned to the image without diffusion weighting to make sure all the diffusion-weighted images were in alignment with each other. The diffusion tensor was calculated for each voxel by using a least-squares fit of the tensor model. Tensor eigenvalues and eigenvectors values were calculated and FA, MD, RD, and AxD were determined per voxel by using DTIFIT in FSL.
To extract the FA, MD, RD, and AxD values for the white matter, the resulting DTI images were aligned to standard space. Normally, these images are aligned to a standard space using the FMRIB58_FA standard-space image that is provided within the TBSS package of the FSL Software. As the mean age of our study population differed greatly from the FMRIB cohort on which the TBSS template brain is based,35 we used the anatomically most representative subject from our dataset as study-specific template brain, using the -n option of tbss_2_reg.36
The standard FSL TBSS procedure was followed. All FA images were nonlinearly transformed to a 1 × 1 × 1 mm3 space. Subsequently, the target image was affine aligned into MNI152 standard space, and every image was transformed into 1 × 1 × 1 mm3 MNI152 space by combining the nonlinear transform to the target FA image with the affine transform from that target to MNI152 space. Only FA was used for registration. White matter was delineated using the FAST tool of FSL and was thresholded at 0.45 to correct for possible partial volume effects. The delineated white matter was transformed to MNI152 standard space using the transformation matrices obtained in the previous step. Subsequently, individual FA, MD, RD, and AxD values were extracted from the TBSS skeleton by calculating the means over all voxels.
EEG recording and post-processing
Details on EEG acquisition and post-processing are described in our previous study.11 In short, EEGs were recorded with 21 Ag/AgCl electrodes at the positions of the International 10–20 System. Based on visual assessment, five high-quality epochs (4096 samples per epoch, i.e., 40.96 s in total) were selected per subject during eyes closed resting state. Data were re-referenced to the source derivation setting in BrainWave (version 0.9.152.4.1,37 available at http://home.kpn.nl/stam7883/brainwave.html). EEG recordings were bandpass filtered into seven frequency bands, among which was the A2 frequency band (10.0–13.0 Hz). Relative A2 power was calculated and functional connectivity was assessed in the A2 frequency with the phase lag index, a measure that indicates the consistency with which one phase is leading or lagging the other phase.37
Statistical analyses
Statistical analyses were performed by using Statistical Package for the Social Sciences (SPSS) version 23.0 for Windows (SPSS Inc., Chicago, IL, USA). Phi-coefficient tests and Mann–Whitney U tests were used to test for differences between the group with data derived from multiple neuromodalities and the rest of the follow-up cohort.
The relation between motor outcome and outcomes of multiple neuromodalities was assessed by calculating the Pearson’s correlation coefficient for variables without outliers and by calculating Spearman’s ρ for variables with one or more outliers. Outliers were defined as those with z-scores >3.29.38 In addition, correction for age during neuromodality data acquisition and sex was performed by calculating partial Pearson’s and Spearman’s correlations. The Benjamini–Hochberg procedure was applied to correct for multiple comparisons and a false discovery rate of 0.05 was used.39 To avoid multicollinearity, we selected the most significant measure per modality for further investigation.
A final model was constructed to determine if the combination of multiple neuromodalities would aid in explaining the observed variance in motor outcome in very preterm born children. Generalized estimating equations (GEE) modeling was chosen to correct for the relatively high number of twins (31%) within the dataset. Univariate regressions were conducted with motor outcome as dependent variable and GA, BW, sex, dexamethasone treatment, BPD, sepsis or NEC, high-grade IVH, and MRI score at TEA as independent variables to reveal possible confounders. Variables from the univariate analyses with p values < 0.05 were separately included in a hierarchical regression model including the three neuromodality outcomes. When this model explained significantly more of the variance (R2), the variable was indicated as a confounder and it was included in the final GEE model. The alpha level was set at α = 0.05.
Results
Description of the cohort and demographics
Seventy-nine of 85 children underwent motor evaluation during follow-up as part of clinical care, as assessed with M-ABC-II-NL. The other 6 of 85 children were not assessed for motor function. Reasons were the existence of severe cerebral palsy (n = 4), impaired vision (n = 1), or incomplete follow-up (n = 1). In 64 of these 79 children, informed consent was obtained and these children underwent MRI and EEG examination at 10.85 (0.49) years of age in addition to motor evaluation at 9.83 (0.70) years of age. The maximal time span between M-ABC-II-NL and the MRI and EEG examination was 2.81 years (median 11.52 months). The group with children who underwent both motor evaluation and MRI/EEG (n = 64) differed from the rest of the follow-up cohort (n = 21) and had a significantly higher GA and BW (GA: U = 875, p = 0.038; BW: U = 941, p = 0.006). For the group of children who underwent motor evaluation (n = 15) and the group of children who underwent both motor evaluation and multiple neuromodality measurements (n = 64), no significant differences were found for sex and MRI score at TEA (Φ = 0.199, p = 0.063 and Φ = 0.231, p = 0.100) and motor scores were similar (U = 565, p = 0.284).
Of the 64 children, 51 volumetric MRI scans, 54 DTI sequences, and 54 EEG recordings were of sufficient quality for analysis (see Fig. 1 for the flowchart of the study population). For 31 children, data of sufficient quality was acquired for all three neuromodalities; these 31 children did not differ from the rest of the original cohort regarding GA, BW, sex, MRI score at TEA, and IVH grade (data not shown). Table 1 shows an overview of the demographic and clinical variables of the study population.
Motor outcome and results of multiple neuromodalities measurements
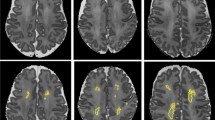
Brain volumes, white matter integrity, and brain function outcomes are summarized in Table 2. The first set of analyses examined the relationship between motor outcome and brain volumes, white matter integrity, and brain function aspects. The results of the correlation analyses are set out in Table 2. The scaled score of the M-ABC-II-NL was significantly correlated to ventricular volume, radial and MD, and relative A2 power and A2 functional connectivity after correcting for age during multiple neuromodalities measurements follow-up and sex and multiple comparison correction. Fig. 2 shows two representative cases: one with low ventricular volume, high A2 power, low RD, and a high motor score, and other with high ventricular volume, low A2 power, high RD, and a low motor score.
Relative A2 power, i.e., the ratio of the area under the curve (AUC) between 10 and 13 Hz and the AUC of the total power spectrum, is visualized by the AUC of A2 power (gray). a Neuromodality data of a male (age 10.74 years) with a high M-ABC-II-NL scaled score of 12. He had high relative A2 power of 12.2%, low ventricular volume (12,315 mm2) as reflected by smaller ventricles in the MR image, and low white matter radial diffusivity (6.07 × 10−4). b Neuromodality data of a male (age 10.20 years) with a low M-ABC-II-NL scaled score of 3. He had a low relative A2 power (5.4%), high ventricular volume (42,793 mm²) as reflected by the large ventricles in the MR image, and high radial diffusivity in the white matter (7.43 × 10−4).
The strongest relationships between scaled M-ABC-II-NL score and outcomes of multiple neuromodalities were selected per modality for the final model, i.e., ventricular volume, RD of the white matter, and relative A2 power.
Univariate regression analyses were performed with possible confounders as independent variables and motor outcome as dependent variable. Results showed that BW (B = 0.003, p = 0.001), BPD (B = −2.22, p = 0.011), MRI score at TEA (B = −2.69, p = 0.001) and dexamethasone treatment (B = −4.30, p < 0.001) were significantly associated to motor outcome.
The possible confounders were subsequently separately included in a hierarchical regression in addition to ventricular volume, RD, and relative A2 power. The addition of BW and dexamethasone treatment resulted in significantly higher explained variance and were therefore included in the final GEE model (BW: R2 change = 0.161, F = 9.75, p = 0.002; dexamethasone treatment: R2 change = 0.184, F = 11.81, p = 0.002). To increase the generalizability of the model, we excluded one outlier (low motor outcome but high relative A2 power and low ventricular volume) that substantially influenced the model, which was also the only SGA born child in the subgroup of children from which all neuromodalities measurements were available for GEE modeling. Table 3 shows the results of the GEE model, based on data from children who underwent both volumetric MRI, DTI, and EEG (n = 31). Ventricular volume and relative A2 power remain significantly associated to motor outcome (B = −9.42 × 10−5, p = 0.027 and B = 28.9, p = 0.007, respectively). The scatterplots of motor outcome with ventricular volume, relative A2 power and RD are shown in Fig. 3.
Discussion
The aim of this study was to determine if the combination of both advanced MRI and EEG techniques would increase the understanding of the observed variance in motor outcome in very preterm born children at school age. Different neuromodalities, providing data on functional and structural brain organization at different spatial and temporal scales, have different neuronal and structural underpinnings and thereby provide us with unique information on the brain.40 The final model, which included the most significant correlation per neuromodality, showed that both the ventricular volume and relative upper alpha power were independently associated with motor outcome. This finding underscores the importance of combining both MRI and EEG techniques, since it suggests that there is a lasting interplay between structure and function that might underlie different motor outcomes in very preterm born children at school age.
Contrary to previous findings, this study was unable to demonstrate significant associations between motor outcome and cerebellar volume and deep gray matter volume, and only a modest association was found between motor score and white matter volume after correcting for total intracranial volume. These inconsistencies may be due to the combination of a modest sample size and a relatively more mature cohort of very preterm infants with a mean GA of 29.1 weeks. These children might exhibit less prominent brain alterations that might only be exposed by the most robust and sensitive measures. This is in agreement with previous literature, which indicated that ventricular volume is the most sensitive biomarker for neurodevelopmental outcome in very preterm infants.41 In addition, the MRI selection process may play a role in the explanation for the inconsistencies; the brains with large focal parenchymal lesions are more difficult to segment and this might have caused a bias in the group toward children with less severe injury.
There is nowadays a considerable amount of literature around the theme of white matter abnormalities in the preterm born population. DTI studies have provided evidence that disrupted white matter integrity is related to adverse motor outcome.23 In agreement with our findings, Thompson et al. found differences in MD, AxD, and RD, but not in FA, between very preterm and term infants,42 which supports the hypothesis that especially mean, axial, and RD are sensitive measures for white matter abnormalities. It is of interest, though, that RD, a measure of white matter integrity, was not independently associated with motor outcome in the final model. This rather contradictory result may be attributed to the variance that is explained by the ventricular volume, which is thought to reflect atrophy of the periventricular white matter.43 The relation between motor outcome and increased ventricular volume has been reported previously in the preterm born population, showing that ventriculomegaly around term age was related with adverse motor outcome at two years of age.44 Although volumetric measures and anisotropy are separate entities, they may measure the same underlying white matter injury that is associated with motor outcome, such as alterations in white matter myelination.45
It has been shown that preterm birth immediately affects brain function and that alterations remain present up to early adolescence.46,47,48 EEG abnormalities have been associated with delayed neuromotor development and can predict non-optimal motor outcomes.49,50 In preterm born children and adolescents, alpha oscillations seem to be an interesting EEG characteristic to study, since lower power, slowing, and altered functional connectivity of alpha oscillations have been related to clinical findings, including motor performance.11,12,51,52
The finding that upper alpha oscillatory power and ventricular volume are independently associated with motor outcome raises questions regarding the link between structural integrity and brain function. The combination of the often-affected white matter in this population and the link between structural connectivity and EEG measures found in this study suggests that there is an interplay between structure and function that underlies adverse outcome. However, this does not explain the independency of ventricular volume and relative upper alpha power. A possible explanation for this discrepancy might be that damaged periventricular white matter can but will not necessarily result in unfavorable brain function, which might be attributed to functional compensatory mechanisms as described in other disease populations.53,54
The findings presented in this study are limited by the small sample size of the study, especially for the GEE model in which the three neuromodalities were combined. We decided to investigate original volumes and volumes as a percentage of the total intracranial volume since it remains unclear if head size is determined by the size of the brain tissue or vice versa. There might be differences between the performance of the studied group and the group of children who were lost to follow-up. This thought is strengthened by the high number of severely affected children in the group in which motor evaluation was not possible. In addition, no control group was included, and it is, therefore, important to interpret preterm birth-related pathophysiological explanations with caution. Further research should be undertaken to explore the links between data derived from multiple neuromodalities and other outcomes as well, including cognitive and behavioral outcomes.
Conclusion
This exploratory study using advanced MRI and EEG techniques offers some insight into how alterations in brain development relate to each other and might support future investigations on motor outcome in prematurely born children. One major finding was the independent association of ventricular volume and relative upper alpha power to motor outcome, supporting the use of ventricular volume and quantitative EEG as study objects in future research. The clinical relevance of these MRI and EEG markers at 9–10 years of age for the prediction of motor outcome after preterm birth seems limited as biomarkers for motor outcome should be available at an earlier age. Nonetheless, the findings support the idea that a combination of neonatal EEG and ventricular volume measured at TEA may improve the prediction of motor outcome, since these measures seem to be the most sensitive measures up to 9–10 years of age. Notwithstanding the relatively limited sample, this work shows the added value of combining data derived from multiple neuromodalities in the preterm born population. Future multiple neuromodalities studies investigating the interplay between brain structure and function in preterm born children are therefore warranted.
Change history
25 June 2021
The name of the the first author was corrected.
References
Helenius, K. et al. Survival in very preterm infants: an International Comparison of 10 National Neonatal Networks. Pediatrics 140, e20171264 (2017).
Parikh, N. A. Advanced neuroimaging and its role in predicting neurodevelopmental outcomes in very preterm infants. Semin. Perinatol. 40, 530–541 (2016).
Taylor, M. J. Structure and function: how to connect? Neuroradiology 55, 55–64 (2013).
Volpe, J. J. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 8, 110–124 (2009).
Anderson, P. J., Cheong, J. L. & Thompson, D. K. The predictive validity of neonatal MRI for neurodevelopmental outcome in very preterm children. Semin. Perinatol. 39, 147–158 (2015).
Mathur, A. M., Neil, J. J. & Inder, T. E. Understanding brain injury and neurodevelopmental disabilities in the preterm infant: the evolving role of advanced magnetic resonance imaging. Semin. Perinatol. 34, 57–66 (2010).
de Kieviet, J. F. et al. Brain development of very preterm and very low-birthweight children in childhood and adolescence: a meta-analysis. Dev. Med. Child Neurol. 54, 313–323 (2012).
Nosarti, C. et al. Grey and white matter distribution in very preterm adolescents mediates neurodevelopmental outcome. Brain 131, 205–217 (2008).
Nosarti, C. et al. Preterm birth and structural brain alterations in early adulthood. Neuroimage Clin. 6, 180–191 (2014).
Whitford, T. J. et al. Brain maturation in adolescence: concurrent changes in neuroanatomy and neurophysiology. Hum. Brain Mapp. 28, 228–237 (2007).
C, van‘tWestende et al. The degree of prematurity affects functional brain activity in preterm born children at school-age: an EEG study. Early Hum. Dev. 148, 105096 (2020).
Twilhaar, E. S. et al. EEG profiles and associated neurodevelopmental outcomes after very preterm birth. Clin. Neurophysiol. 130, 1166–1171 (2019).
Melhem, E. R. et al. Diffusion tensor MR imaging of the brain and white matter tractography. Am. J. Roentgenol. 178, 3–16 (2002).
Beaulieu, C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 15, 435–455 (2002).
Andica, C. et al. MR biomarkers of degenerative brain disorders derived from diffusion imaging. J. Magn. Reson. Imaging. 52, 1620–1636 (2019).
Kwon, S. H. et al. Functional magnetic resonance connectivity studies in infants born preterm: suggestions of proximate and long-lasting changes in language organization. Dev. Med. Child Neurol. 58, 28–34 (2016).
Pandit, A. S., Ball, G., Edwards, A. D. & Counsell, S. J. Diffusion magnetic resonance imaging in preterm brain injury. Neuroradiology 55, 65–95 (2013).
Gano, D. White matter injury in premature newborns. Neonatal Netw. 35, 73–77 (2016).
Huang, H. Structure of the fetal brain: what we are learning from diffusion tensor imaging. Neuroscientist 16, 634–649 (2010).
Xu, D., Mukherjee, P. & Barkovich, A. J. Pediatric brain injury: can DTI scalars predict functional outcome? Pediatr. Radiol. 43, 55–59 (2013).
Pecheva, D. et al. Recent advances in diffusion neuroimaging: applications in the developing preterm brain. F1000Res 7, F1000FacultyRev-1326 (2018).
Li, K. et al. Fractional anisotropy alterations in individuals born preterm: a diffusion tensor imaging meta-analysis. Dev. Med. Child Neurol. 57, 328–338 (2015).
de Kieviet, J. F. et al. A crucial role of altered fractional anisotropy in motor problems of very preterm children. Eur. J. Paediatr. Neurol. 18, 126–133 (2014).
Hinojosa-Rodriguez, M. et al. Clinical neuroimaging in the preterm infant: diagnosis and prognosis. Neuroimage Clin. 16, 355–368 (2017).
Sui, J. et al. Function-structure associations of the brain: evidence from multimodal connectivity and covariance studies. Neuroimage 102, 11–23 (2014).
de Bruine, F. T. et al. Prognostic value of gradient echo T2* sequences for brain MR imaging in preterm infants. Pediatr. Radiol. 44, 305–312 (2014).
Leijser, L. M. et al. Brain imaging findings in very preterm infants throughout the neonatal period: part I. Incidences and evolution of lesions, comparison between ultrasound and MRI. Early Hum. Dev. 85, 101–109 (2009).
Bancalari, E. & Claure, N. Definitions and diagnostic criteria for bronchopulmonary dysplasia. Semin. Perinatol. 30, 164–170 (2006).
Bell, M. J. et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 187, 1–7 (1978).
Hoftiezer, L. et al. From population reference to national standard: new and improved birthweight charts. Am. J. Obstet. Gynecol. 220, 383 e381–383.e317 (2019).
Volpe, J. J. Volpe’s Neurology of the Newborn (Elsevier, 2018).
Kidokoro, H., Neil, J. J. & Inder, T. E. New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. Am. J. Neuroradiol. 34, 2208–2214 (2013).
Dale, A. M., Fischl, B. & Sereno, M. I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9, 179–194 (1999).
Fischl, B., Sereno, M. I. & Dale, A. M. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage 9, 195–207 (1999).
Smith, S. M. et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage 31, 1487–1505 (2006).
Ball, G. et al. An optimised tract-based spatial statistics protocol for neonates: applications to prematurity and chronic lung disease. Neuroimage 53, 94–102 (2010).
Stam, C. J., Nolte, G. & Daffertshofer, A. Phase lag index: assessment of functional connectivity from multi channel EEG and MEG with diminished bias from common sources. Hum. Brain Mapp. 28, 1178–1193 (2007).
Field, A. P. Discovering Statistics Using SPSS (and Sex and Drugs and Rock ‘n’ Roll) (SAGE, 2009).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 57, 289–300 (1995).
Uludag, K. & Roebroeck, A. General overview on the merits of multimodal neuroimaging data fusion. Neuroimage 102, 3–10 (2014).
Keunen, K. et al. Brain volumes at term-equivalent age in preterm infants: imaging biomarkers for neurodevelopmental outcome through early school age. J. Pediatr. 172, 88–95 (2016).
Thompson, D. K. et al. Regional white matter microstructure in very preterm infants: predictors and 7 year outcomes. Cortex 52, 60–74 (2014).
Allin, M. et al. Effects of very low birthweight on brain structure in adulthood. Dev. Med. Child Neurol. 46, 46–53 (2004).
Brouwer, M. J. et al. Sequential cranial ultrasound and cerebellar diffusion weighted imaging contribute to the early prognosis of neurodevelopmental outcome in preterm infants. PLoS ONE 9, e109556 (2014).
Volpe, J. J., Kinney, H. C., Jensen, F. E. & Rosenberg, P. A. The developing oligodendrocyte: key cellular target in brain injury in the premature infant. Int. J. Dev. Neurosci. 29, 423–440 (2011).
Gonzalez, J. J. et al. Assessment of electroencephalographic functional connectivity in term and preterm neonates. Clin. Neurophysiol. 122, 696–702 (2011).
Scher, M. S. et al. Comparisons of EEG spectral and correlation measures between healthy term and preterm infants. Pediatr. Neurol. 10, 104–108 (1994).
Suppiej, A. et al. Spectral analysis highlight developmental EEG changes in preterm infants without overt brain damage. Neurosci. Lett. 649, 112–115 (2017).
Yerushalmy-Feler, A. et al. Electroencephalographic characteristics in preterm infants born with intrauterine growth restriction. J. Pediatr. 164, 756–761.e751 (2014).
Shellhaas, R. A. et al. Neonatal sleep-wake analyses predict 18-month neurodevelopmental outcomes. Sleep 40, zsx144 (2017).
Doesburg, S. M. et al. Magnetoencephalography reveals slowing of resting peak oscillatory frequency in children born very preterm. Pediatr. Res. 70, 171–175 (2011).
Doesburg, S. M. et al. Region-specific slowing of alpha oscillations is associated with visual-perceptual abilities in children born very preterm. Front. Hum. Neurosci. 7, 791 (2013).
Gaubert, S. et al. EEG evidence of compensatory mechanisms in preclinical Alzheimer’s disease. Brain 142, 2096–2112 (2019).
Peterson, D. S. & Fling, B. W. How changes in brain activity and connectivity are associated with motor performance in people with MS. Neuroimage Clin. 17, 153–162 (2018).
Acknowledgements
The Department of Neonatology received a grant from Chiesi Pharmaceuticals B.V. Schiphol, The Netherlands for the execution of this study. We wish to thank R.J.M. Berkhout for her assistance during the data collection of the study. We would also like to thank all the technicians of the laboratory of the Clinical Neurophysiology and Radiology Department of the Leiden University Medical Center. We thank J. Lak and R. van Leeuwen for the assistance with the acquisition of the EEG and MRI data and the guidance of the patients and their parents, and Prof. Dr. Linda de Vries for carefully reviewing the current manuscript. All authors meet the authorship requirements. S.J.S. received a grant from Chiesi Pharmaceuticals B.V. Schiphol, The Netherlands for the execution of this study. Chiesi Pharmaceuticals B.V. was not involved in the conduct of the research.
Author information
Authors and Affiliations
Contributions
C.v.W.: conceptualization, methodology, formal analysis, investigation, writing—original draft, visualization; S.J.S.: conceptualization, data acquisition, methodology, writing—review and editing, supervision, project administration, funding acquisition; L.J.: conceptualization, methodology, writing—review and editing, project administration; A.A.v.d.B.-H.: conceptualization, methodology, formal analysis, investigation, data curation, writing—review and editing L.A.v.d.P.: writing—review and editing, supervision; F.T.W.-d.B.: conceptualization, data acquisition, methodology, writing—review & editing; C.J.S.: software, writing—review and editing, supervision; C.M.P.C.D.P.-S.: conceptualization, data acquisition, methodology, writing—review and editing, supervision, project administration.
Corresponding author
Ethics declarations
Competing interests
C.M.P.C.D.P.-S. is founder and consultant at Neurophyxia BV. She holds several patents and stocks of Neurophyxia BV. None of this work has a relationship with the current manuscript. The other authors declare no competing interests.
Consent statement
Written consent was obtained from both parents, and all children received age-specific information about the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
van ’t Westende, C., Steggerda, S.J., Jansen, L. et al. Combining advanced MRI and EEG techniques better explains long-term motor outcome after very preterm birth. Pediatr Res 91, 1874–1881 (2022). https://doi.org/10.1038/s41390-021-01571-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01571-x