Abstract
For children, there are very few published reviews focusing on severe acute pancreatitis (AP). PubMed, EMBASE, Web of Science, Scopus, Chinese National Knowledge Infrastructure (CNKI), Wanfang data, EBSCO, and Cochrane Library were searched from inception until March 2020. Meta-regression analyses were used to estimate the etiology, case fatality, recurrence, and severity of pediatric AP in different regions (North America, Asia, South America, Europe, and Oceania). Pooled data from 47 papers (48 studies) found that main causes of pediatric AP were gallstones in Asia; trauma in Oceania; and idiopathic in Europe, North America, and South America. The case-fatality rate (CFR) of pediatric AP is 4.7% (North America), 6.2% (Europe), 2.4% (Asia), 3.1% (South America), and 7.4% (Oceania). The incidence rates of recurrent acute pancreatitis (RAP) in children who have had an episode of acute pancreatitis in North American, Asia, and Europe were 15.3, 13.1, and 13.8%, respectively. The incidence of severe acute pancreatitis (SAP) in different regions was 30.3% (Oceania), 29.2% (South America), 20.8% (Europe), 15.8% (Asia), and 13.7% (North America). It suggests that physicians should notice the etiology of pediatric AP for the initial assessment, diagnosis, prediction of relapse, and appropriate treatment at a later stage.
Impact
-
It indicates the etiology of pediatric acute pancreatitis for the initial assessment, diagnosis, and prediction of relapse.
-
Main causes of pediatric AP were gallstones in Asia; trauma in Oceania; and idiopathic in Europe, North America, and South America. The case-fatality rate of pediatric AP is diverse worldwide.
-
It suggests that physicians noticed the etiology of pediatric AP for the initial assessment, diagnosis, prediction of relapse, and appropriate treatment at a later stage.
Similar content being viewed by others
Introduction
Acute pancreatitis (AP) is a rare disease among children,1 characterized by the appearance of inflammatory cells and inducing reversible structural and functional changes within a short duration.2 The yearly incidence of pediatric AP has increased in decades, approximately 3–13 cases per 100,000 children per year.3 Recently, many management strategies were based on evidence in adults, but there was a lack of pediatric-specific management options. It was beneficial to find high-risk patients by knowledge of the etiology of pediatric AP and helpful for interventions to improve treatment by its potential pathogenesis. In a previous study, it was reported that there were obvious differences in the etiology of AP among different regions and AP-associated case-fatality rate (CFR) was rising with age in adults. This rate was rapidly elevated above the age of 59 years.4 But for children, the etiology, case fatality, and recurrence rates of AP were still widely various in the existing publication. In particular, there were very few published reviews focusing on severe acute pancreatitis (SAP) in children. The summary of pediatric AP in different regions about etiology, case fatality, recurrence rates, and SAP was especially lacking. We therefore carried out this systematic review and meta-analysis to estimate the epidemiology of developing pediatric AP in terms of the etiology, case fatality, recurrence, and severity rates in different regions (North America, Asia, South America, Europe, and Oceania).
Methods
Literature search
We conducted this systematic review and meta-analysis according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.5 Eight electronic databases, including PubMed, EMBASE, Web of Science, Scopus, CNKI, Wanfang data, EBSCO, and Cochrane Library, were searched for studies published without language restrictions from database inception to March 2020. The search strategy is detailed in the Appendix Text using terms such as acute pancreatitis, children, pediatric, juvenile, teenager, adolescent, etiology, pathogeny, recurrent, relapse, case fatality, mortality, and death. Titles and abstracts were independently checked for potential suitability by two individuals. We resolved any disagreement of investigators through discussion. Additional search was performed using the bibliographies of the included studies and related systematic reviews.
Selection of studies for inclusion in the review
Studies were eligible with the following characteristics. (1) Pediatric patients aged 0–18 years with AP. (2) Outcome: the epidemiology of the etiology, case fatality, recurrence, or severity rates. (3) To be included, there was a minimum observational size of ten cases for inclusion studies. Exclusion criteria were as follows: (1) only abstract or posters and (2) if multiple studies were retrieved with overlapping cohorts, the latest study was included. Eligibility assessment was conducted by two investigators to check appropriateness for inclusion in the final analysis, with disagreements arbitrated through a third investigator.
Data extraction and quality assessment
Two independent investigators extracted data from each included study, such as author, country, study period, gender, age, and the number of pediatric AP. Severe pancreatitis was defined as a sum of the scores between 6 and 10 points with Ranson’s Criteria.6 The outcomes were assessed via the epidemiology of the etiology, case fatality, recurrence, and SAP in children. For observational studies, the Newcastle–Ottawa Scale (NOS) was used to evaluate bias in studies across three dimensions, including selection, comparability, and outcome. A study was recorded as high quality if it scored 7 out of 9 and medium when the score achieved 5.7
Statistical analysis
Through Stata 12.0 (StataCorp LLC), a random-effects model was used for estimating the incidence rate of case fatality, recurrence, and SAP of pediatric AP in this study due to significant heterogeneity between the studies. The incidence rate of etiology was presented graphically on the y axis and specific etiology on the x axis using the Microsoft Excel 2016 and R softwares. The random-effects meta-regression analyses for outcomes were applied to estimate the potential sources of heterogeneity, for example, age. Between-study heterogeneity was appraised using I2 and Cochran’s Q test.8 The I2 values of 25, 50, and 75% indicated the low, moderate, and high degree of heterogeneity, respectively. Publication bias for exploring small-study effects was assessed by Egger’s test.9 We evaluated the overall certainty in pooled effect estimates using Grading of Recommendations, Assessments, Development and Evaluation (GRADE),10 which estimated quality of evidence by analyzing its risk of bias, imprecision, inconsistency, indirectness, and publication bias.
Results
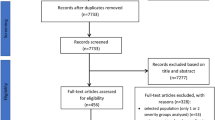
The review process and characteristics of included studies
The search verified 5690 possibly eligible articles. Based on further assessment, 5643 studies were excluded, and the study selection flow diagram is shown in Fig. 1. As a result, a total of 47 papers including 48 studies met the inclusion criteria and were finally enrolled in this meta-analysis.11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57 Table 1 described the characteristics of the included studies. All these selected papers were published after 2005, in which the study period ranged from 1976 to 2017. The sample size for each study varied from 11 to 2127, with a total of 8873 patients.
Risk of bias assessment and certainty of evidence
Through NOS quality assessment, the risk of bias assessment for the included studies is listed in Table 1 and Supplemental Table 1, showing medium-to-high quality with scores of 4–8. Using GRADE approach, the overall certainty of evidence supporting the etiology, case fatality, recurrence, and SAP in pediatric AP was rated moderate.
The etiology of the AP
The main causes of pediatric AP resulted from trauma, systemic disease, alcohol, medication, genetics, gallstones, infection, post-endoscopic retrograde cholangiopancreatography, idiopathic, anatomic anomalies, oncology, and metabolic disease. However, these factors differed among various continents (Fig. 2). The top three causes in different continents were gallstones (33%), systemic disease (31%), and infection (29%) in Asia; trauma (32%), idiopathic (25%), systemic disease (16%), and infection (16%) in Oceania; idiopathic (26%), systemic disease (13%), and infection (13%) in Europe; idiopathic (25%), systemic disease (16%), alcohol (16%), medication (16%), genetics (16%), gallstones (16%), and infection (16%) in North America; idiopathic (29%), medication (19%), and anatomic anomalies (15%) in South America. Gallstones were present in 33% of children with AP in Asia while trauma was the main cause (32%) in Oceania. The main etiology of pediatric AP, accounting for 25–29% of all cases, was idiopathic pancreatitis, which had the highest incidence in Europe, North America, and South America.
The top three causes in different continents were gallstones (33%), systemic disease (31%), infection (29%) in Asia, trauma (32%), idiopathic (25%), systemic disease (16%), infection (16%) in Oceania, idiopathic (26%), systemic disease (13%), infection (13%) in Europe, idiopathic (25%), systemic disease (16%), alcohol (16%), medication (16%), genetics (16%), gallstones (16%), infection (16%) in North America, idiopathic (29%), medication (19%), anatomic anomalies (15%) in South America.
The prevalence of the AP-related death
The overall pooled prevalence of the pediatric AP-related death in children from 30 studies based on 7347 samples was 3.9% (95% confidence interval (CI), 3.5–4.4), with significant heterogeneity present (I2 = 100%; p = 0). According to region groups, they showed various prevalence of pediatric AP-related death as 4.7% (95% CI, 4–5.4), 6.2% (95% CI, 3.7–8.7), 2.4% (95% CI, 1.9–2.9), 3.1% (95% CI, 0.1–6), and 7.4% (95% CI, 5–9.8) in North America, Europe, Asia, South America, and Oceania, respectively (Fig. 3). In meta-regression analysis, after adjusting for correlated incidence data and controlling for ages, it indicated that there was no significant correlation between mean age and CFR of pediatric AP (tau2 = 0.001, p = 0.159; Supplemental Fig. 1). The solid line showed the weighted regression line according to variance-weighted least squares. The inner and outer lines displayed the 95% CI. The area of each circle was proportional to the inverse variance of CFRs (Fig. 3).
The prevalence of pediatric recurrent AP (RAP)
There were 23 studies reporting the prevalence of pediatric ARP, with pooled data of 13.9% (95% CI, 12.5–15.3). According to region groups, they showed similar prevalence of pediatric AP recurrence as 15.3% (95% CI, 12.5–18.1), 13.1% (95% CI, 11.1–15.1), and 13.8% (95% CI, 11.1–16.5) in children who underwent an episode of AP in North America, Asia, and Europe, respectively (Fig. 4). Through univariate meta-regression analysis, a trending positive association (tau2 = 0.006, p = 0.530) was detectable between mean age of study population and recurrence rate (Supplemental Fig. 2).
The prevalence of pediatric SAP
The prevalence of pediatric SAP was reported in 24 studies, with pooled data of 17.2% (95% CI, 15.8–18.6). According to region groups, they had similar prevalence of pediatric SAP as 13.7% (95% CI, 11.5–15.9), 15.8% (95% CI, 13.8–17.8), 29.2% (95% CI, 21.4–37), 20.8% (95% CI, 16.2–25.5), and 30.3% (95% CI, 23.3–37.3) in North America, Asia, South America, Europe, and Oceania, respectively (Fig. 5). Heterogeneity assessment via meta-regression analyses showed that the age was not the main contributor (tau2 = 0.015, p = 0.442) to the high between-study variability (Supplemental Fig. 3).
Assessment of sensitivity analysis and publication bias
No significant changes were detectable based on each outcome when any one study was removed. Egger’s regression asymmetry tests were performed to evaluate publication bias for the prevalence of the case fatality, recurrence, and SAP of pediatric AP. There was significant publication bias in them, with p values of <0.01.
Discussion
The incidence of AP in children is gradually increasing in recent years, which was close to that of adults.58,59 AP could develop into chronic pancreatitis after several months to years. In the late-stage disease, pancreatic structural changes, pain, and pancreatic exocrine or endocrine insufficiency appear to be irreversible, depending on the etiology.60 Moreover, there was also a clear correlation between the cause of AP and case fatality.61 For the different etiology, the treatment of pediatric AP is also different. Therefore, clarifying the etiology of AP is of great importance for the initial assessment, diagnosis, slowing the progression, and treatment.
It was reported that the etiological composition of pediatric AP was different from that of adults. According to the existing reports, the most common causes of pediatric AP included bile or obstructive factors, drugs, and systemic diseases.59 In adults, the leading causes of AP are mainly calculous gall bladder disease and alcohol abuse.62 Furthermore, most causes of AP biliary obstruction are stones or tumors in adults, while in children, they mainly resulted from biliary tract silt.2,59 Meanwhile, the metabolic etiology of children was significantly lower than that of adults. Only 2–7% pediatric AP has metabolic factors.20,63 Recently, the emergence of new drugs, especially the continuous supply of chemotherapeutic drugs, has also led to an increase in the incidence of drug-induced pancreatitis (DIP). It was reported that the incidence of DIP was 0.3–5.3% in recent years,64 but the drug-derived AP accounts for 10–40% in children.59 Therefore, the etiology of adults AP is not suitable for the analysis of pediatric AP. It is urgent to understand the etiology of AP in children.
There are obvious differences in the etiology of pediatric AP among different regions.15,26 This meta-analysis including 47 articles found that the main causes of pediatric AP were gallstones in Asia and trauma in Oceania, idiopathic in Europe, North America, and South America. These findings were conducive to target clinical preliminary assessment and diagnosis of pediatric AP in different races and regions.
The incidence of pediatric SAP in different regions is different. It may have a great relationship with different etiological proportion.61 Studies have shown that idiopathic and hyperlipidemia may easily lead to SAP.61 The analysis of the etiology found that Oceania (30.3%) and South American (29.2%) were idiopathic and metabolic, respectively.
The CFR of pediatric AP in various regions was various. The reason may be that the etiology of pediatric AP varied in different regions. Studies have found that the mortality rate of alcoholic pancreatitis was as high as 30.6%, which was a known cause of high mortality.58 In this study, the two regions with etiology data for alcoholic pancreatitis were North American and Europe. Their CFRs ranked second and third in five continents, respectively.
The focus of treatment of pediatric AP also included prevention of recurrence.65 This study found that the incidence rates of pediatric RAP in North American, Asia, and Europe were 15.3, 13.1, and 13.8%, respectively. Studies on adults found that age was one of the factors associated with rapid progression to RAP during the initial AP attack. However, this study found that there was no correlation between age and pediatric RAP through regression analysis. Some studies reported that genetic mutations (such as PRSS1, SPINK1, CFTR, etc.) were closely related to pediatric RAP.20,66 Therefore, carrying out genetic testing related to RAP in regions with high genetic mutations was vital for the diagnosis and prediction of RAP in children.
There were several limitations that were largely associated with factors in the primary data. First, the heterogeneity between studies was still existing unexplained by the variables examined, which caused major uncertainty around the predicted estimates, may be partly from an issue inherent to AP epidemiology. Second, there was insufficient data for subgroup analyses. Meta-regression analyses were performed to find the potential heterogeneity. Studies differed widely in design, number of patients, and the size of the geographic area covered. Because of the limitations of the included research data, it failed to conduct sex- and gender-based analysis in this meta-analysis, which may result in sex and gender bias.67 Third, small-study effects examined by publication bias may overestimate the effect sizes. Thus, these findings should be read with caution during the interpretation of meta-analyses. Fourth, the longer period ranged (1976–2017) may vary in the AP of diagnosis, and the larger varied sample (11–2127) made a difference to the result. Period ranged and sample sizes could bring instability to the result. Despite these limitations, this meta-analysis offered a comprehensive overview of the prevalence of AP.
In conclusion, the etiology composition of pediatric AP in different regions is various. The epidemiology of recurrence, case fatality, and severity of pediatric AP was varied in diverse regions, which may be related to different etiological factors, but not to the age of children. It would be helpful if physicians pay attention to the etiology of pediatric AP for the preliminary assessment, diagnosis, prediction of relapse, and appropriate treatment at a later stage, with the goal of reducing mortality. Meanwhile, it would be meaningful to clarify the differences in the etiology of pediatric AP in different regions to help health decision-makers understand the epidemiology of the disease in their respective regions and to provide a basis for the implementation of local pediatric AP health policy.
References
Yadav, D. & Lowenfels, A. B. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 144, 1252–1261 (2013).
Bai, H. X., Lowe, M. E. & Husain, S. Z. What have we learned about acute pancreatitis in children? J. Pediatr. Gastroenterol. Nutr. 52, 262–270 (2011).
Morinville, V. D., Barmada, M. M. & Lowe, M. E. Increasing incidence of acute pancreatitis at an American pediatric tertiary care center: is greater awareness among physicians responsible? Pancreas 39, 5–8 (2010).
Marta, K. et al. Aging and comorbidities in acute pancreatitis I: a meta-analysis and systematic review based on 194,702 patients. Front. Physiol. 10, 328 (2019).
Knobloch, K., Yoon, U. & Vogt, P. M. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J. Craniomaxillofac. Surg. 39, 91–92 (2011).
Balthazar, E. J., Robinson, D. L., Megibow, A. J. & Ranson, J. H. Acute pancreatitis: value of CT in establishing prognosis. Radiology 174, 331–336 (1990).
Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25, 603–605 (2010).
Higgins, J. P., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. BMJ 327, 557–560 (2003).
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997).
Balshem, H. et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 64, 401–406 (2011).
Vitale, D. S. et al. Blood urea nitrogen elevation is a marker for pediatric severe acute pancreatitis. Pancreas 48, 363–366 (2019).
Vidal, E., Alberici, I. & Verrina, E. Acute pancreatitis in children on chronic maintenance dialysis. Pediatr. Nephrol. 34, 1501–1512 (2019).
Nauka, P. C. et al. Validation of lipase and systemic inflammatory response syndrome as prognostic indicators in pediatric acute pancreatitis: a retrospective analysis. J. Pediatr. Gastroenterol. Nutr. 68, 389–393 (2019).
Galai, T. et al. Young age predicts acute pancreatitis severity in children. J. Pediatr. Gastroenterol. Nutr. 68, 720–726 (2019).
Cheng, Y. J. et al. Epidemiology of pediatric acute pancreatitis in taiwan: a nationwide population-based study. J. Pediatr. Gastroenterol. Nutr. 68, e7–e12 (2019).
Sweeny, K. F. et al. Rapid progression of acute pancreatitis to acute recurrent pancreatitis in children. J. Pediatr. Gastroenterol. Nutr. 68, 104–109 (2019).
Zheng, W. et al. Amalgamation of systemic inflammatory response syndrome score with C-reactive protein level in evaluating acute pancreatitis severity in children. Scand. J. Gastroenterol. 53, 755–759 (2018).
Sag, E. et al. Acute pancreatitis in children: a single center experience over ten years. Turk. J. Pediatr. 60, 153–158 (2018).
Izquierdo, Y. E. et al. Multivariate model for the prediction of severity of acute pancreatitis in children. J. Pediatr. Gastroenterol. Nutr. 66, 949–952 (2018).
Grzybowska-Chlebowczyk, U. et al. Acute pancreatitis in children. Prz. Gastroenterol. 13, 69–75 (2018).
Alabdulkareem, A. et al. Etiology and clinical characteristics of pediatric acute pancreatitis in Saudi Arabia: a 20-year experience from a single tertiary center. Int. J. Pediatr. Adolesc. Med. 5, 13–17 (2018).
Suzuki, M. et al. Validation of severity assessment for acute pancreatitis in children. Pediatr. Int. 59, 1127–1128 (2017).
Grover, A. S. et al. The utility of the systemic inflammatory respsonse syndrome score on admission in children with acute pancreatitis. Pancreas 46, 106–109 (2017).
Majbar, A. A. et al. Incidence and clinical associations of childhood acute pancreatitis. Pediatrics. 138, e20161198 (2016).
Hashimoto, N. et al. Efficacy of pediatric acute pancreatitis scores at a Japanese tertiary center. Pediatr. Int. 58, 224–228 (2016).
Hao, F., Guo, H., Luo, Q. & Guo, C. Disease progression of acute pancreatitis in pediatric patients. J. Surg. Res. 202, 422–427 (2016).
Bierma, M. J. et al. Predicting severe acute pancreatitis in children based on serum lipase and calcium: a multicentre retrospective cohort study. Pancreatology 16, 529–534 (2016).
Abu-El-Haija, M. et al. Early enteral nutrition in children with acute pancreatitis. J. Pediatr. Gastroenterol. Nutr. 62, 453–456 (2016).
Suzuki, M. et al. Scoring system for the prediction of severe acute pancreatitis in children. Pediatr. Int. 57, 113–118 (2015).
Goday, P. S. et al. Acute pancreatitis in the pediatric intensive care unit. J. Pediatr. Gastroenterol. Nutr. 61, 108–112 (2015).
Bolia, R. et al. Prevalence, natural history, and outcome of acute fluid collection and pseudocyst in children with acute pancreatitis. J. Pediatr. Gastroenterol. Nutr. 61, 451–455 (2015).
Terlizzi, V. et al. Prediction of acute pancreatitis risk based on PIP score in children with cystic fibrosis. J. Cyst. Fibros. 13, 579–584 (2014).
Guo, Q. et al. Predictors for mortality following acute pancreatitis in children. Pediatr. Surg. Int. 30, 1111–1115 (2014).
Boskovic, A. et al. The role of D-dimer in prediction of the course and outcome in pediatric acute pancreatitis. Pancreatology 14, 330–334 (2014).
Antunes, H., Nascimento, J., Mesquita, A. & Correia-Pinto, J. Acute pancreatitis in children: a tertiary hospital report. Scand. J. Gastroenterol. 49, 642–647 (2014).
Kim, S. C. & Yang, H. R. Clinical efficacy of gabexate mesilate for acute pancreatitis in children. Eur. J. Pediatr. 172, 1483–1490 (2013).
Lautz, T. B. et al. Utility of the computed tomography severity index (Balthazar score) in children with acute pancreatitis. J. Pediatr. Surg. 47, 1185–1191 (2012).
Fabre, A. et al. Severity scores in children with acute pancreatitis. J. Pediatr. Gastroenterol. Nutr. 55, 266–267 (2012).
Zhu, Y. M. et al. [Clinical characteristics of children with acute pancreatitis]. Zhonghua Er Ke Za Zhi 49, 10–16 (2011).
Chang, Y. J. et al. Acute pancreatitis in children. Acta Paediatr. 100, 740–744 (2011).
Park, A. et al. Changing referral trends of acute pancreatitis in children: a 12-year single-center analysis. J. Pediatr. Gastroenterol. Nutr. 49, 316–322 (2009).
Li, N. & Wang, X. Y. [Relationship between acute pancreatitis and systemic inflammation response syndrome in children]. Zhongguo Dang Dai Er Ke Za Zhi 10, 715–718 (2008).
Kandula, L. & Lowe, M. E. Etiology and outcome of acute pancreatitis in infants and toddlers. J. Pediatr. 152, 106.e1–110.e1 (2008).
Nydegger, A. et al. Changing incidence of acute pancreatitis: 10-year experience at the Royal Children’s Hospital, Melbourne. J. Gastroenterol. Hepatol. 22, 1313–1316 (2007).
Chen, C. F., Kong, M. S., Lai, M. W. & Wang, C. J. Acute pancreatitis in children: 10-year experience in a medical center. Acta Paediatr. Taiwan 47, 192–196 (2006).
Stringer, M. D. et al. Multidisciplinary management of surgical disorders of the pancreas in childhood. J. Pediatr. Gastroenterol. Nutr. 40, 363–367 (2005).
Laugel, V. et al. [Severe acute pancreatitis in children receiving asparaginase: multicenter retrospective study]. Arch. Pediatr. 12, 34–41 (2005).
Werlin, S. L., Kugathasan, S. & Frautschy, B. C. Pancreatitis in children. J. Pediatr. Gastroenterol. Nutr. 37, 591–595 (2003).
Choi, B. H. et al. Acute pancreatitis associated with biliary disease in children. J. Gastroenterol. Hepatol. 18, 915–921 (2003).
Alvarez Calatayud, G. et al. Acute pancreatitis in childhood. Rev. Esp. Enferm. Dig. 95, 40–44, 45-48 (2003).
Tiao, M. M. et al. Pancreatitis in children: clinical analysis of 61 cases in southern Taiwan. Chang Gung Med. J. 25, 162–168 (2002).
Pezzilli, R. et al. Acute pancreatitis in children. An Italian multicentre study. Dig. Liver Dis. 34, 343–348 (2002).
DeBanto, J. R. et al. Acute pancreatitis in children. Am. J. Gastroenterol. 97, 1726–1731 (2002).
Javid, G. et al. Etiology and outcome of acute pancreatitis in children in Kashmir (India). An endemic area of hepatobiliary ascariasis. World J. Surg. 37, 1133–1140 (2013).
Yeung, C. Y. et al. Pancreatitis in children–experience with 43 cases. Eur. J. Pediatr. 155, 458–463 (1996).
Berney, T. et al. Influence of severe underlying pathology and hypovolemic shock on the development of acute pancreatitis in children. J. Pediatr. Surg. 31, 1256–1261 (1996).
Weizman, Z. & Durie, P. R. Acute pancreatitis in childhood. J. Pediatr. 113, 24–29 (1988).
Uc, A. & Husain, S. Z. Pancreatitis in children. Gastroenterology 156, 1969–1978 (2019).
Husain, S. Z. & Srinath, A. I. What’s unique about acute pancreatitis in children: risk factors, diagnosis and management. Nat. Rev. Gastroenterol. Hepatol. 14, 366–372 (2017).
Steer, M. L., Waxman, I. & Freedman, S. Chronic pancreatitis. N. Engl. J. Med. 332, 1482–1490 (1995).
Zhu, Y. et al. A study on the etiology, severity, and mortality of 3260 patients with acute pancreatitis according to the Revised Atlanta Classification in Jiangxi, China over an 8-year period. Pancreas 46, 504–509 (2017).
Shukla-Udawatta, M., Madani, S. & Kamat, D. An update on pediatric pancreatitis. Pediatr. Ann. 46, e207–e211 (2017).
Restrepo, R. et al. Acute pancreatitis in pediatric patients: demographics, etiology, and diagnostic imaging. AJR Am. J. Roentgenol. 206, 632–644 (2016).
Zheng, J., Yang, Q. J., Dang, F. T. & Yang, J. Drug-induced pancreatitis: an update. Arab J. Gastroenterol. 20, 183–188 (2019).
Pant, C. et al. Acute recurrent pancreatitis in children: a study from the pediatric health information system. J. Pediatr. Gastroenterol. Nutr. 62, 450–452 (2016).
Abu-El-Haija, M. et al. Genetic variants in acute, acute recurrent and chronic pancreatitis affect the progression of disease in children. Pancreatology 19, 535–540 (2019).
Heidari, S. et al. Sex and Gender Equity in Research: rationale for the SAGER guidelines and recommended use. Res Integr. Peer Rev. 1, 2 (2016).
Acknowledgements
This study was supported by the National Key R&D Program of China (2018YFC0114900), the Development Project of National Major Scientific Research Instrument (82027803), the Development Project of National Major Scientific Research Instrument of China (8202780008), the National Natural Science Foundation of China (81971623), the Major Research plan of the National Natural Science Foundation of China (91630311), the National S&T Major Project of China (2018ZX10301201), the Natural Science Foundation of Zhejiang Province (LZ20H180001), and Zhejiang Provincial Association Project for Mathematical Medicine (LSY19H180015).
Author information
Authors and Affiliations
Contributions
Conceptualization: T.J. Acquisition of data: all authors. Analysis and interpretation of data: G.T., L.Z. Writing—original draft: G.T. Critical revision of the manuscript for important intellectual content: T.J. Formal analysis: G.T., Q.Z. Funding acquisition: Q.Z., T.J. Methodology: Q.Z. Supervision: T.J.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Patient consent
Because this is a meta-analysis, patient consent was not required.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tian, G., Zhu, L., Chen, S. et al. Etiology, case fatality, recurrence, and severity in pediatric acute pancreatitis: a meta-analysis of 48 studies. Pediatr Res 91, 56–63 (2022). https://doi.org/10.1038/s41390-021-01454-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01454-1