Abstract
Background
Infants born moderate to late preterm constitute the majority of preterm births, yet guidelines for their nutritional care are unclear. Maternal milk is the most appropriate nutrition for these infants; however, its composition can be influenced by environmental factors. The present study therefore investigated perinatal predictors of human milk composition in a preterm cohort.
Methods
Milk was collected during the DIAMOND trial (DIfferent Approaches to Moderate and late preterm Nutrition: Determinants of feed tolerance, body composition and development) from 169 mothers of 191 infants at three time-points (5 and 10 days post partum and 4 months’ corrected age). Leptin, adiponectin and insulin-like growth factor-1 (IGF-1) were analysed by enzyme-linked immunosorbent assay. Generalised mixed models were used to evaluate associations between milk composition and maternal/infant/perinatal factors.
Results
Most findings were independent of collection time-point. Gestational diabetes was associated with lower adiponectin. Higher adiponectin and lower leptin were associated with higher socioeconomic status, higher maternal education and ability to fully breastfeed at discharge from hospital. Higher leptin was associated with high perceived stress during hospital admission. Milk IGF-1 displayed sex-specific patterns in association with maternal social deprivation.
Conclusion
Maternal, infant and environmental factors during the perinatal period were associated with milk compositional profiles throughout lactation. Further clinical trials should investigate the impact of such changes in terms of long-term infant outcomes.
Impact
-
Human milk is the best nutrition for the infant. However, its composition may be susceptible to alterations determined by pathological conditions mother and infant may face throughout pregnancy and in the perinatal period.
-
This study found that perinatal factors are associated with human milk composition from early to late lactation.
-
If human milk composition throughout lactation is “programmed” during pregnancy or early lactation, infants who were exposed in utero to environmental insults may still be exposed to them during lactation. The impact of human milk compositional alteration on infant growth following perinatal pathological events requires further investigation.
Similar content being viewed by others
Introduction
Globally, >1 in 10 infants are born preterm (<37 weeks of pregnancy), equating to over 15 million preterm births annually.1 Preterm births cause more than one million newborn deaths each year,1 and further increase the risk of death due to other causes, especially from neonatal infections2. Infants born moderate to late preterm (32–36 weeks of gestation) account for >80% of all preterm births.3,4 Although survival rates among these infants are high, these individuals are at an increased risk for adverse outcomes in later life, including metabolic and respiratory disorders such as asthma and cardiometabolic disease.5,6,7,8 Preterm birth is, in many cases, an indicator of adverse conditions in the womb.7 Preterm newborns are then further hindered by the abrupt cessation of in utero nutrient supply, which is difficult to restore efficiently immediately post birth.9 In extremely preterm infants, it is well recognised that this can lead to inadequate early postnatal nutrition,10 but in moderate to late preterm infants, for whom nutritional guidelines are to date not defined,11,12 this has not been described. This nutritional inadequacy can lead to significant rates of postnatal faltering growth13 and altered body composition even by term-corrected age.14
Maternal human milk (HM), with or without fortifiers, is identified as the best nutrition source for moderate and late preterm infants.9,15,16,17 However, HM is well known to be an extremely dynamic and personalised fluid, with a biochemical composition that varies depending upon several inter-18,19,20,21 and intrapersonal22,23,24 factors. We previously have reported in a cohort of healthy pregnancies that HM displayed a characteristic compositional fingerprint in terms of adipokines and growth factors in association with specific maternal, pregnancy and infant characteristics.25 This highly individualised profile underscores the multifactorial nature of HM, which arises as a result of complex interactions between mother, infant and the environment, and suggests its importance in infant outcomes and growth trajectories. In a preterm cohort, the response to the variability of growth factors and adipokines may be even more significant, as these hormones play key roles in functions such as enhancing gut maturation,26,27,28 improving metabolic health29,30 and controlling appetite and gastric emptying,31,32 which are especially important for preterm infants.
Importantly, we and others have demonstrated that HM composition is sex-specific, and important for optimal growth.22 Preterm infants often show sex-specific outcomes,33 and grow at different rates, exemplified by the use of sex-specific growth charts. Therefore, the standardised nutrition preterm infants often receive before breastfeeding is established may not be appropriate. While research on the sex-specific composition of HM is gaining momentum, the potential for sex specificity in preterm HM remains unexplored. Further understanding of the individualised nature of milk is essential for delivering optimal nutrition for preterm infants.
Based on our previous findings, we hypothesised that HM composition in a preterm cohort is associated with perinatal factors, including maternal, infant and environmental conditions.
Materials and methods
Study overview and population
The present research is based on data and HM samples from 191 infants and 169 mothers who took part in the ongoing DIAMOND trial (DIfferent Approaches to Moderate and late preterm Nutrition: Determinants of feed tolerance, body composition and development)34 between May 2017 and June 2019. Infants born between 32+0 and 35+6 weeks of gestation were recruited at four neonatal care units in Auckland, New Zealand (n = 225) and randomised within 24 h of birth to one of three nutrition interventions in a factorial designed trial. Written informed consent was obtained from the parents for the enrolment of their infants. The study protocol was approved by the New Zealand Health and Disability Ethics Committee (16/NTA/90).
While the present study is based on the samples and data coming from the randomised clinical trial, all the analysis have been conducted as for a preterm birth cohort, since the trial is still ongoing and the intervention groups have not been disclosed.
Maternal, pregnancy and infant characteristics
Demographic information from mothers (maternal education, maternal ethnicity and postcode) was collected at recruitment. Postcode information was used to determine the social deprivation index using the New Zealand (NZ) Deprivation Index (1–10, Classification Coding System from Statistics New Zealand35). For analysis, index data were divided into quintiles with Q1 representing the least deprived and Q5 the most deprived areas. Clinical information (gestational diabetes mellitus (GDM), antenatal steroid treatment, mode of birth) and infant sex were obtained from hospital records. Mothers were asked to complete questionnaires to assess the likelihood of postnatal depression (Edinburgh Postnatal Depression Scale, EPDS36,37) and perceived level of stress (Perceived Stress Scale, PSS38) ~10 days after birth and at 4 months’ corrected age (±2 weeks, defined from 40 weeks of gestation). The EPDS referred to how mothers felt during the week prior to completion of the questionnaire. Mothers who scored 10 (maximum score: 30) in the questionnaire possibly experienced depression. The PSS questionnaire referred to how mothers felt during the month prior to completion of the questionnaire. Both questionnaires were self-completed and not obligatory. According to the New Zealand Ministry of Health, the current diagnostic criteria for GDM is fasting glucose ≥5.5 mmol/l or glucose ≥9.0 mmol/l 2 h following a 75 g oral glucose tolerance test.
Feeding practices
Information on nutritional intake in hospital and mode of feeding at discharge was collected. Parents completed a questionnaire at the 4-month follow-up concerning breastfeeding and weaning practices (introduction of food other than HM, introduction of solids, formula consumed by the infant per day). Breastfeeding is defined as the infant feeding only maternal HM at the breast. Bottle feeding is defined as the infant receiving either formula or HM through a bottle. Tube feeding is defined as the infant receiving either formula or HM through an enteral tube. Fully breastfeeding at discharge from hospital/home was defined as the infant being able to feed maternal HM at the breast without further tube or bottle top up. Infants discharged from hospital were not always discharged home; some infants were discharged to peripheral birthing units to establish breast/suck feeds.
HM sample collection
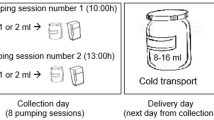
As part of the trial, mothers were asked if they wished to give HM samples at different time-points (day 5 + 2 days, day 10 ± 2 days and 4 months ± 2 weeks’ corrected age) for compositional analysis. The present study included mothers who gave ≥1 HM sample and their infants. A detailed explanation of the collection method has been published elsewhere.39 Samples were collected in the morning between 10 a.m. and 12 p.m., and 2–3 h after the previous expression or breastfeed. Collection was undertaken using hospital-grade breast pumps (Medela, Baar, Switzerland) exclusively from the right breast. Mothers expressed until they felt the breast had been emptied. A total of 2 ml was taken from the expressed sample by using a sterile enteral syringe (different brands as provided by each neonatal unit) and aliquoted into low protein-binding microtubes (Eppendorf, Hamburg, Germany). Samples were stored at −80 °C until analysis.
HM analyses
Leptin, adiponectin and insulin-like growth factor-1 (IGF-1) analyses were performed using commercially available ELISA (enzyme-linked immunosorbent assay) kits (human-sensitive leptin ELISA, human adiponectin ELISA and human IGF-1 ELISA; Mediagnost, Reutlingen, Germany) as previously described.25 HM total protein was quantified in order to normalise the hormone concentrations and to investigate the associations with maternal–infant factors. Protein quantification was performed via infrared spectrometry using the Direct Detect® technology (Merck, Germany).25 Samples were randomised for all assays.
Statistical analyses
Concentrations of HM bioactives were corrected for total protein concentration in each sample (mg/ml) and reported as ng/mg of protein per ml (ng/mg). Concentrations of bioactives were assessed for normality through the Shapiro–Wilk test, and as distributions were not normal, they were normalized by log 10 transformation. Protein concentration in HM was normally distributed across mothers and reported as mg/ml. Correlations between compounds and between time-points were investigated via simple linear regression. Linear mixed models were used to investigate differences in HM bioactive concentrations over time (from day 5 to 4 months’ corrected age) across the groups defined by nutritional practices (fully breastfeeding at discharge from hospital, milk and feeding type at discharge home, age at the introduction of solids and ml/day of formula consumed by the infant at 4 months’ corrected age), socioeconomic indicators (maternal education, NZ Deprivation Index), pregnancy characteristics (administration of antenatal steroids, gestational diabetes, mode of birth and multiple/single pregnancy), maternal stress at discharge and 4 months’ corrected age (PSS and EPDS scores) and infant characteristics (sex, gestational age, weight, length and head circumference z-scores at birth). Each mixed model comparing HM composition over time across the above mentioned groups, with exception of the nutritional practices, was corrected for the ml/day of formula consumed by the infant at 4 months’ corrected age. This correction was applied since the amount of formula consumed by the infant at 4 months was significantly correlated to the change in HM protein concentration across time. Sex-specific interactions and variations across time-points were investigated by including infant sex and time as independent variables in each model (i.e. three independent variables per model were tested, and one cofactor). Bonferroni correction was applied when comparing multiple groups. The p value in the main text reflects the analysis of the main effects, while the p values displayed in the table refer to post hoc analyses. All statistical analyses were performed using IBM SPSS (version 25) and the graphs were generated by using GraphPad Prism 8. The power provided by the sample size of the population for simple and multiple linear regression and repeated-measure analysis was calculated with G*Power 3.1.9.2 (University of Düsseldorf) as >90% at the 5% significance level for the detection of 10% difference across groups for all the measured main effects.
Results
Population characteristics
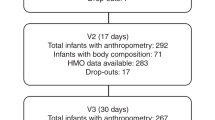
The participant and sample numbers are detailed in Fig. 1 and the demographic characteristics of the population provided in Table 1. The majority (74%) of participants was recruited in Auckland City Hospital and Middlemore Hospital neonatal care units, and Caucasian was the most represented ethnicity, followed by Asian, Pacific Islanders and Māori. Over half of the mothers delivered via caesarean section and <20% were diagnosed with GDM. Males accounted for over half of the infants, and ~20% of all infants were twins.
HM hormones
Supplementary Fig. 1 shows a summary of HM hormonal concentrations across the time-points in the study population. All of the analytes significantly changed across the three time-points (p < 0.0001 for each). Leptin and IGF-1 concentrations were significantly correlated to each other at days 5 and 10 (Supplementary Fig. 2). No other correlation between hormone concentrations was found in this cohort.
Time interactions
Antenatal steroid treatment was associated with higher HM total protein concentrations on day 5, particularly when mothers received the treatment >7 days prior to giving birth (Fig. 2). No other associations presented were impacted by sample time-point.
N none, I incomplete, C complete, O over 7 days before birth, MC multiple courses. The X in a indicates that no mothers in the MC group provided a sample at the 4 months’ corrected age visit. The shading bars on the right of each heatmap indicate the concentrations of HM protein. The lighter the colour the lower the concentration. P values refer to interaction effects. Data are expressed as estimated marginal means (±SEM), obtained from each fitted model (generalised mixed model; main factors: time, sex and the factor on the x-axis; cofactor: ml/day of formula consumed by the infant at 4 months’ corrected age).
Feeding practices and HM composition
Most infants were able to fully breastfeed at discharge from hospital and only fed maternal HM at discharge home (Supplementary Table 1). At 4 months’ corrected age, at least 45% were breastfeeding and at least 26% were not consuming any formula. HM of mothers whose infants were not fully breastfed at discharge from hospital had higher IGF-1 and leptin concentrations and lower adiponectin concentrations, compared to those whose infants were fully breastfed (Supplementary Table 2). In addition, mothers whose infants consumed greater amounts of formula by the 4 months’ corrected age (ml/day) had overall higher HM protein concentrations independent of time of collection (p = 0.002).
Pregnancy, maternal and infant characteristics and HM composition
Leptin concentrations were higher (p = 0.036) and adiponectin concentrations were lower (p = 0.010) in the most deprived quintile compared with the least deprived quintile (Table 2). Maternal ethnicity was also associated with differences in HM leptin (p = 0.024) and adiponectin (p = 0.001) with Māori and Pacific Island groups having lower adiponectin concentrations than Caucasians. Singleton vs. multiple pregnancy was associated with HM leptin (p = 0.020), with singletons receiving milk with higher leptin. In addition, GDM was associated with significantly lower HM adiponectin (p < 0.001). C-section was associated with lower HM leptin (p = 0.007) and lower IGF-1 (p = 0.005). Gestational age was associated with HM adiponectin, with mothers who gave birth at 35 weeks’ gestation having higher HM adiponectin compared to the other groups and specifically to mothers who gave birth at 33 weeks’ gestation. Finally, higher HM leptin was observed in mothers who reported high stress the month prior to discharge (p = 0.002).
Sex-specific HM composition
Sex-specific differences in HM were observed for IGF-1 in association with NZ deprivation index quintiles, with HM for male and females containing significantly different IGF-1 in the third (Q3, IGF-1 higher for male infants) and fifth (Q5, IGF-1 higher for female infants) quintiles (Fig. 3). Sex-specific effects were also evident in association with HM protein in GDM (higher protein for males compared to females) vs. non-GDM mothers (p = 0.036). No other significant sex-specific interactions were observed in this cohort.
Sex-specific interactions shown (all time-points analysed) are a HM composition and NZ Deprivation Index and b HM composition and GDM. Open circles/bars=female infant; Closed circles/bars=male infant. Note female and male symbols overlap for Q1 and Q2 in a. IGF-1 insulin-like growth factor-1. *p < 0.05 between the two sexes. The data represent estimated marginal means (±SEM) obtained by each fitted generalised mixed model (main factors: time, sex, NZ Deprivation Index/GDM; cofactor: ml/day of formula consumed by the infant at 4 months’ corrected age).
Discussion
In the present study, we examined the associations between maternal–infant factors and HM composition in a preterm cohort. Our results confirm that HM composition is associated with maternal characteristics, pregnancy, infant and environmental factors. Sex-specific HM composition was observed in association with the socioeconomic status of the family, confirming previous evidence.40 Perinatal factors (GDM, antenatal steroid treatment and perceived stress during hospital admission) showed significant associations with HM composition independent of time of collection.
We observed an increase in leptin and IGF-1 concentrations from early lactation to 4 months corrected age. Evidence in relation to the fluctuations of leptin and IGF-1 over time in HM have been limited and conflicting, likely due to the different approaches to HM sampling protocols and cohort characteristics.41,42,43 Some studies report absolute hormone concentrations (per ml of HM), while in the present cohort hormone concentrations were normalised to protein content. As high protein concentrations characterize HM from mothers of preterm infants, particularly during early lactation,44 concentrations of IGF-1 and leptin in our cohort are lower on days 5 and 10 after birth. Previous reports have suggested a slight decline in HM leptin over the period of early lactation up to 3 months of age with concentrations then remaining relatively stable until 6 months post partum41. When similarly expressed as ng/ml, our leptin data align with this observation with a slight decrease in concentration from day 5 and 10 until 4 months’ corrected age. Moderate increases in HM IGF-1 concentrations during early lactation have been reported in both term and preterm infants43 and this is confirmed with our data, whether adjusted for protein or expressed as ng/ml. Mature HM concentrations of IGF-1 in this cohort are very similar to mature HM concentrations of IGF-1 as reported by our group previously in a healthy Finnish cohort.25
HM hormone composition was most strongly related to feeding practices and socioeconomic indicators. These two factors appeared to be strongly linked to each other and to maternal ethnicity, in our population. We observed that mothers of infants who were not fully breastfeeding at hospital discharge had decreased HM adiponectin and increased HM leptin compared to mothers of infants who were fully breastfeeding. Similarly, mothers living in more deprived areas displayed lower adiponectin and higher leptin than mothers living in the higher quintile. This relationship is partially explained by the fact that significantly more infants from less socially deprived areas were fully breastfed at discharge. Yet, adjusting the analyses by the NZ Deprivation Index only slightly attenuated the significance of the results. Previous studies have found that education and income were strongly associated with breastfeeding practices.45 While we did not have any information on family income, families with less income are more likely to inhabit more socially deprived areas.46
In addition, mothers with higher education were more likely to feed their infant with only HM and their infants were more likely to breastfeed at discharge. Interestingly, a HM composition (lower adiponectin and higher leptin) similar to the one observed for mothers living in more deprived areas, and less likely to fully breastfeed their infant at discharge from hospital, was also observed in mothers with lower education. Comparable results were found in relation to maternal ethnicities, with Caucasians displaying a higher percentage of fully breastfed infants at discharge compared to all other ethnicities, and higher HM adiponectin and lower HM leptin, especially compared to Māori and Pacific Islanders. Altogether, these findings are consistent with social disparities across the different ethnicities living in New Zealand47 and suggest that the socioeconomic status of the family plays an important role in the factors that determine HM composition. The fact that adjusting these analyses for NZ Deprivation Index attenuated but did not abolish this relationship suggests that other factors, including perhaps maternal diet and overall health status, which are typically related to the socioeconomic status of the family, contribute to HM composition and breastfeeding practices.
Our results also suggest an association between protein concentration during early lactation and ability to establish HM supply or maintain lactation throughout time. For instance, the overall amount of total protein in HM (all time-points considered) was significantly associated with the ml/day of formula consumed by the infant at 4 months’ corrected age, with mothers of infants consuming more formula around the 4th month of follow-up visit having higher HM protein. It is interesting to note that in the same cohort, mothers of infants who were exclusively formula fed at discharge also had significantly higher total protein in their HM on day 5, and especially on day 10, and did not provide a sample at the 4th month visit. While only five infants were exclusively formula fed at discharge and we can only assume that infants consuming more formula at 4 months’ corrected age were those that consumed less HM, previous studies have reported on the utility of HM total protein as a biomarker for lactation performance.48,49 Such studies demonstrated how the transition from colostrum in small amounts to that of abundant mature HM, known as lactogenesis II, is typically defined by changes in maternal metabolism and reflected in changes in the composition of HM. The amount of total protein has been identified as one of four biomarkers for the initiation of lactogenesis II.50 As HM protein should decrease within the first 5 days after birth,51 elevated protein concentrations for a prolonged time after birth are likely to indicate a delay in the phase of lactogenesis II, which is considered a major determinant for early cessation of breastfeeding.52 As the use of lactation biomarkers would be extremely useful in promptly addressing lactation issues, researchers have recently validated it for pump-dependent mothers of preterm infants.53 The study found that mothers of preterm infants (<33 weeks’ gestation) presented altered composition of one or more of the four biomarkers during the first 14 days post partum and that this alteration was associated with lower milk volumes and difficulty in establishing HM supply. While we did not measure lactose, sodium and citrate in our samples, it is interesting to note that in our cohort, mothers with higher protein in their HM were more likely to feed their infants with more formula by 4 months’ corrected age or to prematurely stop breastfeeding. This observation strengthens the hypothesis that mothers who have high protein in their HM at day 5 post partum and after might experience difficulties in establishing and/or maintaining milk supply. Although the higher amount of protein in HM can be due to mothers expressing less frequently and/or expressing less volume of HM, after correcting the analysis for total volume of milk expressed at collection (on days 5 and 10) and frequency of expression in the 24 h prior to collection (on days 5 and 10) mothers who fed their infant exclusively with formula at discharge home had still significantly higher protein in their milk on day 10 (24 vs. 16 mg/ml on average in all other groups). Furthermore, our results show that antenatal steroid treatment, another factor that causes difficulties in establishing HM supply after preterm birth,54 was also associated with higher HM protein. Mothers who received any antenatal corticosteroids before birth displayed increased HM protein on day 5 post partum compared to those who did not receive any.
In line with our previous study,25 data from the present cohort suggest that HM composition may be subject, in part, to perinatal programming mechanisms, which most likely involves signalling deriving from mother, environment and infant. For instance, HM composition showed significant sex-specific differences. This impacted on HM IGF-1, with mothers living in mildly deprived areas (quintile 3) producing milk with higher IGF-1 for males and mothers living in highly deprived areas (quintile 5) producing milk with higher IGF-1 for females. This is consistent with the finding from Fujita et al.40 of HM sex specificity in settings where the socioeconomic status of mothers is low. According to the Trivers–Willard hypothesis, mothers invest more on their female infants in poverty and more on their male infants in wealth;55 however, from our findings it is hard to define whether this is the case in our cohort. Nonetheless, it would appear that that mothers living in lower socioeconomic status environments may produce HM, which is different for male and female infants. More specific studies should investigate whether this relationship simply manifests an act of evolutionary selection/pressure or suggests that in settings where infants may struggle the most to thrive, maternal HM is even more personalised to confer greater support. Further sex-specific interactions were observed in relation to GDM and total protein concentration in HM. GDM mothers of males produced HM with significantly higher protein compared to GDM mothers of females and non-GDM mothers. We previously observed sex-specific interactions in association with GDM and HM adiponectin,25 and GDM has been previously reported to alter the proteomic profile of HM.21,56,57 While higher protein intake is desired for preterm infants,58 high protein consumption during early life is significantly associated with greater risk of obesity during childhood.59 Furthermore, irrespective of infant sex, HM of GDM mothers had lower adiponectin concentrations compared to non-GDM mothers at all time-points. In both this and our previous study, HM alterations due to GDM appear to be limited to male infants. This might have implications for postnatal outcomes related to cardiometabolic disease in male infants born to GDM mothers, as we know that adiponectin is an important factor in the regulation of inflammation and cardiometabolic function.60 Yet, we still do not know if HM hormonal concentrations are correlated with changes in infant circulating hormones, and this should be addressed by future studies that also look at the impact of altered HM composition on longer-term health outcomes. In support of the hypothesis that perinatal stressors may persist into postnatal life exposing the infant to suboptimal stimuli, we also observed that higher HM leptin was associated with high perceived stress. According to previous research, leptin is involved in the stress response61 and increases following glucocorticoid release in acute stress episodes.62 In this case, we found that the association between stress and higher leptin concentrations in HM were not limited to short time periods and acute responses, suggesting that the stress mothers underwent during the perinatal period may be linked to HM composition throughout lactation. Taken together, these findings suggest that HM composition is associated with maternal, infant and environmental components, reiterating HM multifactorial nature. Alterations in HM composition have been observed following pregnancy and perinatal stressors (GDM, moderate/high stress, antenatal steroid treatment). While it is currently unknown whether such alterations are positive or negative for the infant, this is a crucial information to gather in order to have a clearer picture of how pregnancy and perinatal hardship might translate into the long term.
The major limitation of the present study was the lack of data around some maternal factors, including prepregnancy BMI, reason for preterm delivery and diet, which may have contributed to some of the associations with socioeconomic status, education and ethnicity. In addition, only 38% of mothers provided a sample at 4 months’ corrected age, hence affecting the evaluation of longitudinal changes in milk composition. The study was overall very well powered, with the exception of the subgroup of infants exclusively formula fed at discharge, which only included five infants. HM collection was also standardised as much as feasible, with very similar collection timeframes and conditions for all mothers in order to ensure comparability across samples.
The findings from this study are consistent with our previous work25 and confirm the complex nature of the HM signature. Furthermore, by being able to assess the variation in HM composition over a distinct period of time (from 5 days post partum to 4 months’ corrected age), the present study strengthens the hypothesis that the influence of events and conditions that arise during pregnancy and early lactation on HM composition persist throughout the lactation period. This highlights the need for further research into the role of pregnancy complications and environmental influences on the establishment and compositional profile of HM. Future studies should investigate whether these factors increase the morbidity risk for infants and, if so, whether lifestyle or dietary intervention in lactating mothers may help reverting them. Our results further confirm previous literature that suggest the use of total protein as a biomarker for the early identification of lactation difficulties mothers may face in a preterm setting and that often results in early cessation of breastfeeding.
References
Blencowe, H. et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 379, 2162–2172 (2012).
Lawn, J. E., Kerber, K., Enweronu-Laryea, C. & Cousens, S. 3.6 Million neonatal deaths—what is progressing and what is not? Semin. Perinatol. 34, 371–386 (2010).
Frey, H. A. & Klebanoff, M. A. The epidemiology, etiology, and costs of preterm birth. Semin. Fetal Neonatal Med. 21, 68–73 (2016).
Shapiro-Mendoza, C. K. & Lackritz, E. M. Epidemiology of late and moderate preterm birth. Semin. Fetal Neonatal Med. 17, 120–125 (2012).
Crump, C., Winkleby, M. A., Sundquist, K. & Sundquist, J. Risk of diabetes among young adults born preterm in Sweden. Diabetes Care 34, 1109–1113 (2011).
Machado, L. C., Passini Júnior, R. & Rodrigues Machado Rosa, I. Late prematurity: a systematic review. J. Pediatr. 90, 221–231 (2014).
Shapiro-Mendoza, C. K. et al. Effect of late-preterm birth and maternal medical conditions on newborn morbidity risk. Pediatrics 121 e223–32. (2008).
Crump, C., Winkleby, M. A., Sundquist, K. & Sundquist, J. Risk of hypertension among young adults who were born preterm: a Swedish national study of 636,000 births. Am. J. Epidemiol. 173, 797–803 (2011).
Tudehope, D. I. Human milk and the nutritional needs of preterm infants. J. Pediatr. 162, S17–S25 (2013).
Cormack, B. E., Harding, J. E., Miller, S. P. & Bloomfield, F. H. The influence of early nutrition on brain growth and neurodevelopment in extremely preterm babies: a narrative review. Nutrients 11, 2029 (2019).
Alexander, T. & Bloomfield, F. H. Nutritional management of moderate–late preterm infants: survey of current practice. J. Paediatr. Child Health 55, 338–342 (2019).
Lapillonne, A. et al. Feeding the late and moderately preterm infant. J. Pediatr. Gastroenterol. Nutr. 69, 269–270 (2019).
Iacobelli, S. et al. Nutrition practice, compliance to guidelines and postnatal growth in moderately premature babies: the NUTRIQUAL French survey. BMC Pediatr. 15, 110 (2015).
Olhager, E. & Törnqvist, C. Body composition in late preterm infants in the first 10 days of life and at full term. Acta Paediatr. 103, 737–743 (2014).
Gianni, M. L., Roggero, P. & Mosca, F. Human milk protein vs. formula protein and their use in preterm infants. Curr. Opin. Clin. Nutr. Metab. Care 22, 76–81 (2018).
Abrams, S. A., Schanler, R. J., Lee, M. L. & Rechtman, D. J. Greater mortality and morbidity in extremely preterm infants fed a diet containing cow milk protein products. Acad. Breastfeed. Med. 9, 281–285 (2014).
Quigley, M. & McGuire, W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst. Rev. 22, CD002971 (2014).
Panagos, P. G. et al. Breastmilk from obese mothers has pro-inflammatory properties and decreased neuroprotective factors. J. Perinatol. 36, 284–290 (2016).
Pundir, S. et al. Maternal influences on the glucocorticoid concentrations of human milk: the STEPS study. Clin. Nutr. 38, 1913–1920 (2018).
Purcell, R. H. et al. Maternal stress and high-fat diet effect on maternal behavior, milk composition, and pup ingestive behavior. Physiol. Behav. 104, 474–479 (2011).
Klein, K. et al. Concentration of free amino acids in human milk of women with gestational diabetes mellitus and healthy women. Breastfeed. Acad. Breastfeed. Med. 8, 111–115 (2013).
Galante, L. et al. Sex-specific human milk composition: the role of infant sex in determining early life nutrition. Nutrients 10, 1194 (2018).
Pundir, S. et al. Variation of human milk glucocorticoids over 24h period. J. Mammary Gland Biol. Neoplasia 22, 85–92 (2017).
Coppa, G. V. et al. Changes in carbohydrate composition in human milk over 4 months of lactation. Pediatrics 91, 637–641 (1993).
Galante, L. et al. Sexually dimorphic associations between maternal factors and human milk hormonal concentrations. Nutrients 12, 152 (2020).
Ma, L. & Xu, R. J. Oral insulin like growth factor-I stimulates intestinal enzyme maturation in newborn rats. Life Sci. 61, 51–58 (1997).
Kimble, R. M., Breier, B. H., Gluckman, P. D. & Harding, J. E. Enteral IGF-I enhances fetal growth and gastrointestinal development in oesophageal ligated fetal sheep. J. Endocrinol. 162, 227–235 (1999).
Hirai, C. et al. Trophic effect of multiple growth factors in amniotic fluid or human milk on cultured human fetal small intestinal cells. J. Pediatr. Gastroenterol. Nutr. 34, 524 (2002).
Yamauchi, T. et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 8, 1288–1295 (2002).
Ouchi, N. et al. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class a scavenger receptor expression in human monocyte-derived macrophages. Circulation 103, 1057–1063 (2001).
Picó, C. et al. The intake of physiological doses of leptin during lactation in rats prevents obesity in later life. Int. J. Obes. 31, 1199–1209 (2007).
Cannon, A. M. et al. The relationship of human milk leptin and macronutrients with gastric emptying in term breastfed infants. Pediatr. Res. 82, 72–78 (2017).
Stevenson, D. K. et al. Sex differences in outcomes of very low birthweight infants: the newborn male disadvantage. Arch. Dis. Child. Fetal Neonatal Ed. 83, F182–F185 (2000).
Bloomfield, F. H. et al. The DIAMOND trial—DIfferent Approaches to MOderate & late preterm Nutrition: determinants of feed tolerance, body composition and development: Protocol of a randomised trial. BMC Pediatr. 18, 220 (2018).
Atkinson J, Salmond C, Crampton P. NZDep2013 Index of Deprivation. (Department of Public Health, University of Otago, Wellington, 2014).
Cox, J. L., Holden, J. M. & Sagovsky, R. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry 150, 782–786 (1987).
Wisner, K. L., Parry, B. L. & Piontek, C. M. Postpartum depression. N. Engl. J. Med. 347, 194–199 (2002).
Cohen, S., Kamarck, T. & Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 24, 385 (1983).
Galante, L. et al. Feasibility of standardized human milk collection in neonatal care units. Sci. Rep. 9, 14343 (2019).
Fujita, M. et al. In poor families, mothers’ milk is richer for daughters than sons: a test of Trivers–Willard hypothesis in agropastoral settlements in Northern Kenya. Am. J. Phys. Anthropol. 149, 52–59 (2012).
Schuster, S., Hechler, C., Gebauer, C., Kiess, W. & Kratzsch, J. Leptin in maternal serum and breast milk: association with Infants’ body weight gain in a longitudinal study over 6 months of lactation. Pediatr. Res. 70, 633–637 (2011).
Elmlinger, M. W. et al. Insulin-like growth factors and binding proteins in early milk from mothers of preterm and term infants. Horm. Res. 68, 124–131 (2007).
Ozgurtas, T. et al. Vascular endothelial growth factor, basic fibroblast growth factor, insulin-like growth factor-I and platelet-derived growth factor levels in human milk of mothers with term and preterm neonates. Cytokine 50, 192–194 (2010).
Gidrewicz, D. A. & Fenton, T. R. A systematic review and meta-analysis of the nutrient content of preterm and term breast milk. BMC Pediatr. 14, 216 (2014).
Heck, K. E., Braveman, P., Cubbin, C., Chávez, G. F. & Kiely, J. L. Socioeconomic status and breastfeeding initiation among california mothers. Public Health Rep. 121, 51–59 (2006).
Carter, K. & Imlach Gunasekara, F. Dynamics of Low Income and Deprivation in New Zealand, 2002–2009. Public Health Monograph Series No. 24 (Department of Public Health, University of Otago, Wellington, 2012).
Stats NZ. Wealth patterns across ethnic groups in New Zealand. http://archive.stats.govt.nz/browse_for_stats/people_and_communities/Networth/ethnicity.aspx (2016).
Kulski, J. K. & Hartmann, P. E. Changes in human milk composition during the initiation of lactation. Aust. J. Exp. Biol. Med. Sci. 59, 101–114 (1981).
Cregan, M. D., De Mello, T. R., Kershaw, D., McDougall, K. & Hartmann, P. E. Initiation of lactation in women after preterm delivery. Acta Obstet. Gynecol. Scand. 81, 870–877 (2002).
Kent, J. C. How breastfeeding works. J. Midwifery Women’s Health 52, 564–570 (2007).
Boss, M., Gardner, H. & Hartmann, P. Normal human lactation: closing the gap. F1000Research 7, F1000 (2018).
Hurst, N. Recognizing and treating delayed or failed lactogenesis II. J. Midwifery Women’s Health 52, 588–594 (2007).
Hoban, R. et al. Human milk biomarkers of secretory activation in breast pump-dependent mothers of premature infants. Breastfeed. Med. 13, 352–360 (2018).
Henderson, J. J., Hartmann, P. E., Newnham, J. P. & Simmer, K. Effect of preterm birth and antenatal corticosteroid treatment on lactogenesis II in women. Pediatrics 121, e92–e100 (2008).
Trivers, R. L. & Willard, D. E. Natural selection of parental ability to vary the sex ratio of offspring. Science 179, 90–92 (1973).
Grapov, D. et al. The human colostrum whey proteome is altered in gestational diabetes mellitus. J. Proteome Res. 14, 512–520 (2015).
Smilowitz, J. T. et al. Human milk secretory immunoglobulin A and lactoferrin N-glycans are altered in women with gestational diabetes mellitus. J. Nutr. 143, 1906–1912 (2013).
Tonkin, E. L., Collins, C. T. & Miller, J. Protein intake and growth in preterm infants. Glob. Pediatr. Health 1, 2333794X1455469 (2014).
Weber, M. et al. Lower protein content in infant formula reduces BMI and obesity risk at school age: follow-up of a randomized trial. Am. J. Clin. Nutr. 99, 1041–1051 (2014).
Berg, A. H., Combs, T. P. & Scherer, P. E. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol. Metab. 13, 84–89 (2002).
Tomiyama, A. J. et al. Leptin concentrations in response to acute stress predict subsequent intake of comfort foods. Physiol. Behav. 107, 34–39 (2012).
Trayhurn, P. & Bing, C. Appetite and energy balance signals from adipocytes. Philos. Trans. R. Soc. Ser. B 361, 1237–1249 (2006).
Acknowledgements
We thank the research nurses and the clinical staff for their help with recruitment, data and sample collection. This work was supported by the Health Research Council of New Zealand, Counties Manukau Health and the Liggins Institute.
Author information
Authors and Affiliations
Consortia
Contributions
L.G. drafted the collection protocol for human milk samples, assisted with sample and data collection, performed laboratory work, data analysis and result interpretation and wrote the first draft of the manuscript. C.M.R. and A.M.M. provided input in the design of the collection and laboratory protocols. F.H.B. and T.A. designed the randomised controlled trial, provided funding for sample and data collection and together with D.C.-S. contributed to the collection protocol. M.H.V. provided funding for sample analysis and together with S.P. provided oversight of the process from analysis through to manuscript submission. All authors critically reviewed and edited the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Statement of consent
Written informed consent was obtained from all parents for the enrolment of their infants in the DIAMOND trial and from all mothers who provided human milk samples.
Clinical trial registration
Australian New Zealand Clinical Trials Registry - ACTRN12616001199404.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Galante, L., Reynolds, C.M., Milan, A.M. et al. Preterm human milk: associations between perinatal factors and hormone concentrations throughout lactation. Pediatr Res 89, 1461–1469 (2021). https://doi.org/10.1038/s41390-020-1069-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-1069-1