Abstract
Background
Oxygen and continuous positive airway pressure (CPAP) are primary modes of respiratory support for preterm infants. Animal models, however, have demonstrated adverse unintended effects of hyperoxia and CPAP on lung development. We investigate the effects of combined neonatal hyperoxia and CPAP exposure on airway function and morphology in mice.
Methods
Newborn mice were exposed to hyperoxia (40% O2) 24 h/day for 7 consecutive days with or without daily (3 h/day) concomitant CPAP. Two weeks after CPAP and/or hyperoxia treatment ended, lungs were assessed for airway (AW) hyperreactivity and morphology.
Results
CPAP and hyperoxia exposure alone increased airway reactivity compared to untreated control mice. CPAP-induced airway hyperreactivity was associated with epithelial and smooth muscle proliferation. In contrast, combined CPAP and hyperoxia treatment no longer resulted in increased airway reactivity, which was associated with normalization of smooth muscle and epithelial proliferation to values similar to untreated mice.
Conclusions
Our data suggest that the combination of CPAP and hyperoxia decreases the adverse consequences on airway remodeling of either intervention alone. The complex interaction between mechanical stretch (via CPAP) and hyperoxia exposure on development of immature airways has implications for the pathophysiology of airway disease in former preterm infants receiving non-invasive respiratory support.
Impact
-
CPAP and mild hyperoxia exposure alone increase airway reactivity in the neonatal mouse model.
-
In contrast, combined CPAP and hyperoxia no longer induce airway hyperreactivity.
-
Combined CPAP and hyperoxia normalize smooth muscle and epithelial proliferation to control values.
-
Interaction between CPAP-induced stretch and mild hyperoxia exposure on immature airways has important implications for airway pathophysiology in former preterm infants.
Similar content being viewed by others
Background
Despite marked improvement in the mortality of infants born preterm, respiratory morbidity in this population remains excessive. Chronic pulmonary insufficiency of prematurity after preterm birth persists in this population through infancy, childhood, and beyond.1 In extremely low birth weight infants, it usually begins with a diagnosis of bronchopulmonary dysplasia [BPD], although optimal diagnostic criteria for BPD remain elusive.2 Over the past decade, there has been a marked and successful shift from invasive to non-invasive ventilatory support in neonatal intensive care; however, meta-analysis incorporating four large clinical trials failed to demonstrate significant decrease in BPD in infants exposed to a continuous positive airway pressure (CPAP) versus intubation strategy.3
Assessment of respiratory function in former preterm infants during childhood is typically focused on history of wheezing disorders and measurement of airway (AW) function. Doyle evaluated airflow at 8 years of age in three historical cohorts of extremely preterm infants.4 Between 1997 and 2005, there was a significant increase in days on CPAP accompanied by a non-significant decrease in days of endotracheal intubation.4 There was no accompanying improvement in AW function over this period; in fact, percentage of predicted value of forced expiratory volume in 1 s worsened from 92.0 ± 15.7 to 85.4 ± 14.4, p < 0.05 between the 1997 and 2005 cohorts, respectively. The authors speculated that increased duration of supplemental oxygen during CPAP may have been contributory. Although primarily a problem of infants born very preterm, increased airway reactivity (AWR) is also a problem in moderately preterm or late preterm infants.5,6,7 It is likely that a significant percentage of these infants would also have been exposed to supplemental oxygen delivered via non-invasive positive pressure [CPAP or nasal cannula]. As recently concluded by Been et al., research now needs to focus on understanding underlying mechanisms for the increased risk of asthma in survivors of preterm birth.5
Over the recent years, we have begun to explore the physiologic consequences of neonatal CPAP or supplemental O2 exposure on later AW function by developing a survivable mouse pup model. In our prior study, neonatal mice were exposed to intermittent CPAP delivered via facial mask from postnatal days 1–7 followed by 2-week recovery. We documented significant small AW hyperreactivity at 3 weeks employing an in vitro lung slice preparation.8 This raised the question whether CPAP might be a contributor to the subsequent AW hyperreactivity manifest by preterm human infants. Although non-invasive positive AW pressure is often administered to preterm infants in room air, low supplement oxygen usually accompanies CPAP. Reyburn employed our neonatal mouse CPAP model to study the effects of combined CPAP and 50% oxygen over the first week on respiratory system mechanics.9 The combination of CPAP and hyperoxia reversed the elevated baseline respiratory system resistance (Rrs) induced by hyperoxic exposure. This led us to hypothesize in the current study that, while CPAP and hyperoxia might individually increase AWR in our model, combined exposure may modify these effects and allow us to begin to explore the underlying mechanisms.
Methods
Time-pregnant mice [C57BL/6J] were purchased from a commercial vendor [Charles River] and were later observed to give birth in our animal facility. Mice were treated with or without CPAP in 21 or 40% O2 for the first week of postnatal life; at 3 weeks of postnatal age [P21] [i.e., 2 weeks after CPAP or hyperoxia treatment ended], the lungs were removed in preparation for the measurement of AWR using the precision lung slice preparation. All procedures were carried out in accordance with the National Institutes of Health [NIH] guidelines for care and use of laboratory animals and were approved by the Animal Care and Use Committee at Case Western Reserve University.
Neonatal CPAP and hyperoxia exposure
Following the day of birth [P0], a dam and her litter were randomly assigned to receive either room air [21% O2] or hyperoxia [40% O2] exposure for 7 consecutive days. This level of hyperoxia was chosen based on our prior study demonstrating the effects of various levels of O2 on lung morphology and AWR.10,11 In addition, within a given litter, both male and female pups were then randomly assigned to receive either CPAP [6 cm H2O] or no CPAP starting the following day [P1] for the first 7 postnatal days. The pups and the dam were maintained in a temperature-controlled room during a 12:12 h, light:dark cycle and provided food and water ad lib. Each day, the mice were removed from the dam and fitted with a custom made mask to fit snuggly around the head for administering CPAP.8,9 CPAP was administered to unanesthetized mice, while placed on a temperature-controlled heat pad [Gaymar T/pump, NY] for temperature support. The mask was designed with an entry port to deliver a flow of humidified gas, which passed through to an exit port to a downstream manometer and an adjustable leak; the leak allowed fine-tuning of the level of CPAP while maintaining flow through the mask. CPAP was maintained at 6 cm H2O by adjusting the downstream leak or the upstream flow into the mask. CPAP lasted just 2 h for the first day to minimize time spent away from the nursing dam but was increased to 3 h/session for the following 6 consecutive days [7 days total]. Control mice were also removed from the dam, fitted with the same masks, placed on the same heat pad, received the same airflow via the same tubing, but did not receive CPAP. These control animals breathed the same humidified gas source, but the tubing from the mask was disconnected from the circuit to prevent any risk of backpressure that may arise by virtue of resistance potentially imposed by the excurrent tubing. After each bout of CPAP, the mask was removed and the pups were returned to the mother to resume normal rearing. At the end of the 7 days of CPAP, the mice were allowed an additional 2 weeks of uninterrupted maternal care. These groups comprised room air-exposed control [no CPAP] and room air CPAP-treated animals. Two other groups were treated the same way as the room air-treated animals described above, with the exception that they received 40% O2 for the first postnatal week. Hyperoxia exposure was achieved by placing the dam and her pups in a 38 L Plexiglas chamber with a continuous flow of 40% O2 [4 L/min] for first 7 postnatal days. Hyperoxia was achieved by mixing air and 100% oxygen at appropriate amounts to achieve the desired level of oxygen, which was monitored daily [MiniOX I; MSA Medical]. Hyperoxia-exposed mice that were designated to receive CPAP were removed from the hyperoxia chamber and immediately placed on a mask that also delivered 40% oxygen while the mice were on the mask to receive CPAP as described above. After the seventh day of exposure, the pups and nursing dam were removed from hyperoxia and allowed to develop normally for an additional 14 days in room air. AWR [and tissue collection, see below] was assessed at P21 [i.e., 2 weeks after treatment] using the in vitro living lung slice preparation, as described previously.8,10
Measurement of AWR
At P21, mice were sacrificed via anesthetic overdose [intraperitoneal injection of a ketamine/xylazine mix (100 mg/kg/10 mg/kg, respectively)] to prepare the lungs for in vitro measurement of AWR to methacholine. After death was confirmed, the mouse was placed supine for cannulation of the trachea using a syringe loaded with agarose. The cannula [PE tubing, diameter: 0.5 mm (P8) or 0.58 mm (P21)] was inserted through a small ventral incision through the neck and into the anterior most part of the trachea. The cannula was advanced approximately 3 mm and held securely in place with suture. Liquefied agarose [40 °C] was gently injected to inflate the lungs [0.8 mL] and the mouse was placed in the refrigerator for 30 min to allow the agarose to cool and gel. The entire lung was then removed, placed on a vibratome, sliced into 300 μm sections, and immersed in Dulbecco’s Modified Eagle Medium + Pen/Strep solution for overnight incubation [5% CO2; 37 °C]. The following day, the lung slices were then rinsed in Hanks’ Balanced Salt Solution (HBSS) and mounted in an in vitro recording chamber for live imaging of AW responses to increasing doses of methacholine.
For live imaging of individual AWs, the slices were covered with a thin, lightweight sheet of mesh and a coverslip, which were held in place with silicone grease. The recording chamber containing the slice was mounted on a microscope [DMLFS, Leica Microsystems, Wetzler, Germany] and perfused [7 mL/min] continuously with HBSS at room temperature using a perfusion pump [MPII, Harvard Apparatus, Holliston, MA]. The microscope mounted with a camera [Rolera Fast, QImaging, Canada] was used to identify individual AWs under ×5 magnification. After an initial 3-min period of baseline, the chamber was perfused with increasing doses of methacholine and changes in AW lumen area were recorded continuously. The extent of AW constriction in response to increasing doses of methacholine [0.25, 0.5, 1, 2, 4, and 8 µM] was determined at the end of a 2-min period of exposure to each dose. Image analysis software [ImageJ] was used to determine the luminal area [in pixels] at each dose of methacholine to determine the extent of AW constriction. The greater the decrease in luminal area, the more reactive the AWs.
Individual AWs were chosen at random and the response to methacholine was performed on one AW/lung section, although we typically collected 2–3 sections per animal. Thus treatment groups consisted typically of 2–3 AWs/animal spread across 2–3 litters. Measurements of contractility were assessed only on AWs <0.05 mm2 in lumen area since we showed previously that CPAP effects on AWR were size dependent.8
AW morphology
Lungs from the four groups were inflation fixed [25 cm H2O] for 10 min with 4% paraformaldehyde, embedded in paraffin, and sectioned at 5 μm. One set was stained with hematoxylin and eosin, and AWs were identified under light microscopy (Nikon Ti2; 20X Plan Fluor lens; QImaging 12 bit camera). Images were captured and analyzed with the Nikon Elements software. AW epithelial thickness was measured and averaged over six points per AW. For consistency, three AWs of comparable size per lung were selected. Another set of sections was de-paraffinated and immunolabeled with rabbit anti-alpha smooth muscle myosin antibody [1:5000; Sigma] and Cy3 anti-rabbit antibody. Images were captured on the Nikon microscope with a Photonics high-sensitivity camera. The fluorescently tagged area in the mesenchymal layer was measured and normalized to the diameter of the AW.
Proliferating cell nuclear antigen (PCNA) immunohistochemistry
Lungs from the four groups were inflation fixed [25 cm H2O] for 10 min with 4% paraformaldehyde. The 5-μm paraffin-embedded tissue was treated with 3% H2O2 after antigen retrieval. Lung sections were incubated 24 h at 4 °C with rabbit anti-PCNA antibody [1:10,000; Abcam, Cambridge, MA]. The secondary antibody was biotinylated goat anti-rabbit [1:1000 Jackson ImmunoResearch West Grove, PA, USA], followed by ABC reagent incubation and visualization by diaminobenzidine [Vectastain, Vector Laboratories, Burlingame, CA, USA]. Finally, lung sections were dehydrated and mounted with permount. Primary antibody was omitted for negative controls. All images were obtained using a Rolera XR CCD camera [Q-Imaging, Canada] mounted on a Leica DMLB microscope [Leica Microsystems, Germany]. Images were analyzed using the ImageJ 1.47t software [NIH, Bethesda, USA]. The numbers of labeled PCNA-positive cells were counted in 4–5 AWs at ×20 magnification from 5 to 6 animals/group.
Data analysis
Statistical comparison of responses to methacholine between control and CPAP/hyperoxia-treated groups was made using a two-way, repeated-measures analysis of variance (ANOVA). Comparison of AW morphology parameters between groups was made using two-way ANOVA. Differences were considered significant at p < 0.05. All values for AW lumen area are expressed as mean ± SEM of all AWs and [where relevant] presented as a fraction of initial baseline value, although individual data points are also shown. Sample sizes were based on prior power analysis using the Sigmstat/Sigmaplot software using input values based on our prior studies.
Results
AWR to in vitro methacholine
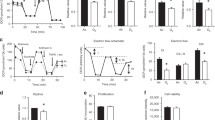
Seven days of neonatal CPAP (RA-CPAP) increased AW constriction to bath-applied methacholine at P21 days compared to untreated control mice (RA-Con; Fig. 1a). Similarly, 7 days of neonatal hyperoxia exposure alone (O2-Con) also caused a long-term increase in AWR compared to RA-Con, which was similar in magnitude to RA-CPAP-treated mice. In contrast, however, when CPAP was administered simultaneously with hyperoxia (O2-CPAP) for the first postnatal week, AWR was significantly reduced compared with either CPAP or hyperoxia treatment alone (Fig. 1a). In fact, AWR in O2-CPAP treated mice was not different from untreated (RA-Con) mice. There was no difference in baseline AW size between the treatment groups (Fig. 1b)
Note, AW reactivity (a) was increased in mice pretreated with CPAP (RA-CPAP) or hyperoxia (O2-Con) alone compared to untreated control mice (RA-Con); however, airway hyperreactivity was no longer present in mice that received combined hyperoxia with CPAP (O2-CPAP). Values are expressed as fraction of baseline lumen size; smaller lumen size at increasing concentration to methacholine signifies increased AW reactivity. Untreated control animals represent animals raised in room air without CPAP treatment (RA-Con). Baseline starting AW lumen area (in image pixels) is also provided to demonstrate studies were performed on airways of equal size between groups (b). Hash (#) indicates that slope of the response for a given treatment group is significantly different from RA-Con. (N = 7–10 airways from 4 to 5 mice/group).
AW epithelial thickness
Seven days of neonatal CPAP (RA-CPAP) increased AW epithelial thickness at P21 days compared to RA-Con mice (Fig. 2a), demonstrating a long-term effect on epithelial morphology. Similarly, hyperoxia exposure also increased epithelial thickness compared to O2-Con. Combined hyperoxia and CPAP treatment (O2-CPAP) also caused an increase in AW epithelial thickness and was similar in magnitude to either CPAP or hyperoxia alone (Fig. 2a). Representative images of AWs from all the four treatment groups are also provided (Fig. 2b).
Treatment groups include untreated mice (no CPAP raised in room air, RA-Con; open black squares), CPAP exposure alone (RA-CPAP, solid squares), hyperoxia exposure alone (O2-Con, open gray squares), and combined CPAP with hyperoxia (O2-CPAP, solid gray squares). Values are mean ± SEM; individual squares represent values for individual animals. Asterisk (*) indicates significantly different from the RA-Con group). Representative images are also shown (b). (N = 6–8 airways from 3 to 4 mice/group).
Airway smooth muscle (ASM) area
Neonatal CPAP (RA-CPAP) increased smooth muscle area at P21 days compared to RA-Con mice (Fig. 3a), demonstrating a long-term effect on ASM morphology. Hyperoxia exposure tended to increase smooth muscle area, although this was not statistically significantly different from RA-Con mice. Interestingly, in contrast to the RA-CPAP group, smooth muscle area was no longer increased in the combined O2-CPAP treatment group (Fig. 3a). Representative images of AWs from all the four treatment groups are also provided (Fig. 3b).
Treatment groups include untreated mice (no CPAP raised in room air, RA-Con; open black squares), CPAP exposure alone (RA-CPAP, solid squares), hyperoxia exposure alone (O2-Con, open gray squares), and combined CPAP with hyperoxia (O2-CPAP, solid gray squares). Values are mean ± SEM; individual squares represent values for individual animals. Asterisk (*) indicates significantly different from the RA-Con group). Representative images are also shown (b). (N = 6–8 airways from 3 to 4 mice/group).
PCNA+ ASM and epithelial cells
As a nuclear marker for identifying cells in a proliferative state, we quantified the number of ASM and epithelial cells stained positively with PCNA. CPAP alone increased the number of both ASM (Fig. 4a) and epithelial cell (Fig. 4b) PCNA+ cells compared to RA-Con mice, whereas hyperoxia alone had no effect. Combined CPAP and O2 exposure prevented the CPAP-induced increase in PCNA+ cells. Representative images are provided in Fig. 4c.
Note the increased number of PCNA+ smooth muscle (a) and epithelium (b) cells in CPAP treated mice compared to untreated control mice. Treatment groups include untreated mice (no CPAP raised in room air, RA-Con; open black squares), CPAP exposure alone (RA-CPAP, solid squares), hyperoxia exposure alone (O2-Con, open gray squares), and combined CPAP with hyperoxia (O2-CPAP, solid gray squares). Values are mean ± SEM; individual squares represent values for individual animals. Asterisk (*) indicates significantly different from the RA-Con group). Representative images are also shown (c). (N = 5–6 airways from 3 to 4 mice/group). Inset: negative control.
Discussion
While the etiology of the chronic lung injury to which preterm infants are predisposed remains multifactorial, supplemental oxygen and intermittent positive pressure delivered via an endotracheal tube are widely implicated.12 It has been difficult to limit hyperoxic exposure to the immature respiratory system; however, non-invasive ventilatory techniques, including CPAP, are now widely employed as an alternative to endotracheal intubation.3,4 This has motivated our research group to explore the effects of supplemental oxygen and the addition of mask CPAP on subsequent AW function in a neonatal mouse model. Our prior in vivo studies demonstrated that hyperoxia-induced increase in baseline Rrs11 was no longer apparent after combined exposure to 50% oxygen and CPAP.9 Therefore, we sought to employ the in vitro lung slice preparation to characterize AWR after individual and combined hyperoxia and CPAP exposures and began to explore the underlying mechanisms.
Numerous investigators have studied the role of hyperoxic exposure in eliciting lung injury in neonatal rodent models, although the focus on AW function after milder degrees of hyperoxia has been somewhat limited.13,14 We have previously used the in vitro living lung slice preparation to document an increase in methacholine-induced AW contraction after a 7-day exposure to 40% supplemental oxygen followed by a 2-week normoxic recovery.10 This was accompanied by a decrease in radial alveolar counts and an increase in expression of AW α-smooth muscle actin after hyperoxic exposure10 although increase in smooth muscle area did not reach significance in the current study.
We have used our novel model of neonatal CPAP administered daily to neonatal mice over the first week of life. CPAP clearly increased AW contractility in the lung slices after 2 weeks of normoxic recovery. We have previously shown that CPAP administered under normoxic conditions did not affect baseline Rrs in mouse pups.9 While baseline Rrs cannot be measured in the slice preparation, the currently observed increase in lung slice contractility was consistent with our prior data.8 In the current study, the accompanying increase in ASM area is consistent with this physiologic effect of CPAP.
Our data clearly demonstrate that the marked AW hyperreactivity induced individually by mild [40%] hyperoxia and CPAP is eliminated when CPAP is applied in the presence of hyperoxia. These findings suggest that CPAP and hyperoxia increase ASM contractility via different mechanisms, or their combined effects would be synergistic. Hyperoxic exposure has been clearly shown to induce proinflammatory mechanisms in the neonatal lung,15 which in turn, is a likely contributor to the parenchymal lung injury induced by hyperoxia. Our prior data have demonstrated a decrease in lung macrophage infiltration after hyperoxia when there is concurrent CPAP exposure,9 which could contribute to our current observations. We speculate that CPAP-induced stretch and resultant ASM and epithelial proliferation may somehow contribute to normalization of the apparent adverse effects imposed by either treatment alone. Our current data are consistent with recent evidence demonstrating intermittent CPAP limits severe hyperoxia-induced lung damage in a rabbit model of BPD,16 although the effects of CPAP alone were not studied. They observed a decrease in Rrs when CPAP was superimposed on hyperoxia, which is consistent with our prior study.9 Gie et al. also observed a significant attenuation of hyperoxic-induced changes in epithelial morphology when CPAP was combined with superimposed hyperoxia. They speculated that the benefits of CPAP resulted from as yet unknown mechanisms that mediate the biological transduction of physical stretch. Although alterations in alveolarization may impact AW function,17 Gie et al. could not attribute the benefit of CPAP superimposed on hyperoxia on alteration in alveolar structure.
Conclusions
Our findings may have potential translational significance for the respiratory morbidity exhibited by former preterm infants.4,5 There has been a marked increase in the use of various forms of non-invasive ventilation for preterm infants, although the ability of those devices to deliver CPAP is not readily quantifiable.18,19 Many of these modes of CPAP delivery, including nasal cannulae, are administered for prolonged periods of time to support lung volume and upper AW patency at a time when supplemental oxygen is minimal or has been discontinued. It is possible that such prolonged CPAP delivery to an immature but relatively normal lung may be an antecedent to later AW hyperreactivity and chronic obstructive pulmonary disease. This may be aggravated by sustained administration of high levels of CPAP. We recognize that data obtained in neonatal rodents may not translate to preterm human infants. Nonetheless, our findings support our hypothesis that combined CPAP and mild hyperoxic exposure attenuate the individual adverse effects of either CPAP or hyperoxia exposure alone.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Steinhorn, R. et al. Chronic pulmonary insufficiency of prematurity: developing optimal endpoints for drug development. J. Pediatr. 191, 15.e1–21.e1 (2017).
Poindexter, B. B. et al. Comparisons and limitations of current definitions of bronchopulmonary dysplasia for the prematurity and respiratory outcomes program. Ann. Am. Thorac. Soc. 12, 1822–1830 (2015).
Schmölzer, G. M. et al. Non-invasive versus invasive respiratory support in preterm infants at birth: systematic review and meta-analysis. BMJ 347, f5980 (2013).
Doyle, L. W. et al. Ventilation in extremely preterm infants and respiratory function at 8 years. N. Engl. J. Med. 377, 329–337 (2017).
Been, J. V. et al. Preterm birth and childhood wheezing disorders: a systematic review and meta-analysis. PLoS Med. 11, e1001596 (2014).
Harju, M. et al. The burden of childhood asthma and late preterm and early term births. J. Pediatr. 164, 295.e1–299.e1 (2014).
Colin, A. A., McEvoy, C. & Castile, R. G. Respiratory morbidity and lung function in preterm infants of 32 to 36 weeks’ gestational age. Pediatrics 126, 115–128 (2010).
Mayer, C. A., Martin, R. J. & MacFarlane, P. M. Increased airway reactivity in a neonatal mouse model of continuous positive airway pressure. Pediatr. Res. 78, 145–151 (2015).
Reyburn, B. et al. The effect of continuous positive airway pressure in a mouse model of hyperoxic neonatal lung injury. Neonatology 109, 6–13 (2016).
Onugha, H. et al. Airway hyperreactivity is delayed after mild neonatal hyperoxic exposure. Neonatology 108, 65–72 (2015).
Wang, H. et al. Severity of neonatal hyperoxia determines structural and functional changes in developing mouse airway. Am. J. Physiol. Lung Cell. Mol. Physiol. 307, L295–L301 (2014).
Higgins, R. D. et al. Bronchopulmonary dysplasia: Executive Summary of a Workshop. J. Pediatr. 197, 300–308 (2018).
Belik, J., Jankov, R. P., Pan, J. & Tanswell, A. K. Chronic O2 exposure enhances vascular and airway smooth muscle contraction in the newborn but not adult rat. J. Appl. Physiol. 94, 2303–2312 (2003). (1985).
Denis, D. et al. Prolonged moderate hyperoxia induces hyperresponsiveness and airway inflammation in newborn rats. Pediatr. Res. 50, 515–519 (2001).
O’Reilly, M. A., Marr, S. H., Yee, M., McGrath-Morrow, S. A. & Lawrence, B. P. Neonatal hyperoxia enhances the inflammatory response in adult mice infected with influenza A virus. Am. J. Respir. Crit. Care Med. 177, 1103–1110 (2008).
Gie, A. G. et al. Intermittent CPAP limits hyperoxia-induced lung damage in a rabbit model of bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell. Mol. Physiol. 318, L976–l987 (2020).
O’Reilly, M., Harding, R. & Sozo, F. Altered small airways in aged mice following neonatal exposure to hyperoxic gas. Neonatology 105, 39–45 (2014).
Claure, N. & Bancalari, E. Non-invasive ventilation in premature infants. Arch. Dis. Child. Fetal Neonatal Ed. 100, F2–F3 (2015).
McEvoy, C. T. et al. Bronchopulmonary dysplasia: NHLBI Workshop on the Primary Prevention of Chronic Lung Diseases. Ann. Am. Thorac. Soc. 11, S146–S153 (2014).
Acknowledgements
We would like to acknowledge the commitment from Morgan Hazard for assistance with CPAP exposure. This study was funded by the National Heart, Lung and Blood Institute (Bethesda, MD) Grants R01HL138402 and R01HL056470 and the Department of Pediatrics, Rainbow Babies and Children’s Hospital, Cleveland, Ohio. This study was also funded in part by generous financial contributions from William and Lois Briggs.
Author information
Authors and Affiliations
Contributions
P.M.M., C.A.M., A.J., C.M.P., and Y.S.P. made substantial contributions to data acquisition and analysis; all authors provided technical and data interpretation expertise; P.M.M. and R.J.M. contributed to conception and experimental design and drafted the manuscript; and all authors revised it critically for intellectual content and approval of the submitted and final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All procedures were carried out in accordance with the National Institute of Health (NIH) guidelines for care and use of laboratory animals and were approved by the Animal Care and Use Committee at Case Western Reserve University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
MacFarlane, P.M., Mayer, C.A., Jafri, A. et al. CPAP protects against hyperoxia-induced increase in airway reactivity in neonatal mice. Pediatr Res 90, 52–57 (2021). https://doi.org/10.1038/s41390-020-01212-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-01212-9
This article is cited by
-
Calcium-sensing receptor and CPAP-induced neonatal airway hyperreactivity in mice
Pediatric Research (2022)