Abstract
Background
Community-acquired pneumonia (CAP) is the leading cause of child deaths around the world. Recently, the vitamin D receptor (VDR) gene has emerged as a susceptibility gene for CAP.
Objectives
To evaluate the association of the VDR gene Fok I polymorphism with susceptibility to CAP in Egyptian children.
Methods
This was a multicenter case-control study of 300 patients diagnosed with CAP, and 300 well-matched healthy control children. The VDR Fok I (rs2228570) polymorphism was genotyped by PCR-restriction fragment length polymorphism (RFLP), meanwhile serum 25-hydroxy vitamin D (25D) level was assessed using ELISA method.
Results
The frequencies of the VDR FF genotype and F allele were more common in patients with CAP than in our control group (OR = 3.6; (95% CI: 1.9–6.7) for the FF genotype; P = 0.001) and (OR: 1.8; (95% CI: 1.4–2.3) for the F allele; P = 0.01). Patients carrying the VDR FF genotype had lower serum (25D) level (mean; 14.8 ± 3.6 ng/ml) than Ff genotype (20.6 ± 4.5 ng/ml) and the ff genotype (24.5 ± 3.7 ng/ml); P < 0.01.
Conclusion
The VDR gene Fok I (rs2228570) polymorphism confers susceptibility to CAP in Egyptian children.
Similar content being viewed by others
Introduction
Community-acquired pneumonia (CAP) is one of the most serious pediatric infections, with an estimated annual incidence to be 33 cases per 1000 children in developed countries. CAP is more common, more severe and is the number one killer of children in the developing world.1 A significant number of CAP patients develop systemic inflammatory response syndrome (SIRS) and sepsis.2 Lung injury resulting in acute respiratory failure (ARF) is the primary complication of pneumonia. Environmental and genetic interplay may affect molecular and cellular processes that cause lung injury in CAP.3 One such environmental factor is vitamin D, as sun exposure is its major source, but it is also provided by some foods. Vitamin D is a group of fat-soluble seco-steroids, plays a crucial role for bone and mineral homeostasis. 1, 25-dihydroxyvitamin D (1, 25D) serves as the active form of vitamin D and binds to the vitamin D receptor (VDR) expressed at the nuclei of target tissues.4 Other than bone, VDR is found on the majority of immune cells; suggesting that vitamin D could modulate both innate and adaptive immunity and also regulate the inflammatory cascade.5 Recent studies have shown that vitamin D and its receptor cast as a pleiotropic regulator of human physiology involved in modification of immune competence, cancer chemoprevention, cardio-protection, propensity to autoimmune diseases and infectious disease risk.6
Vitamin D receptor signaling induces the expression of antimicrobial peptides (AMPs) such as cathelicidin (LL-37), β-defensin, and toll-like receptors (TLRs), molecules important for innate defense against both bacterial and viral infections owing to their chemotactic action and endotoxin neutralization.5,7 At the same time, (1, 25D) can interfere with the instruction of an adaptive immune response pathway, by down-regulating the signals of transcription factors as NF-κB and exerting an anti-inflammatory effect.8 An earlier study by Muhe et al.9 reported that clinical vitamin D deficiency was associated with 13-fold increased risk of CAP in Ethiopian children. Furthermore, inadequate vitamin D stores in children have also been linked to severity and outcome of the disease particularly in the Middle East.10 A randomized controlled trial performed in Kabul concluded that children with pneumonia who received a single high-dose of oral vitamin D3 were less likely to have a repeat episode in the ninety days after supplementation.11
Despite these reports, final proof of a causal role of vitamin D status in CAP is still lacking. A genetic approach could be the only way to clarify this issue. The human VDR gene maps to chromosome 12q13. Although >470 single nucleotide polymorphisms (SNP) have been identified, only a few of them modulate vitamin D uptake. Four polymorphisms of this gene (FokI, BsmI, ApaI, and TaqI) have been the most studied.12
The FokI polymorphism creates an alternative start codon in exon 2 which leads to transcription of two VDR proteins with different structure i.e., a long variant (f-VDR) or a short one (F-VDR).12 The FokI polymorphism has a unique role as it is not in linkage-disequilibrium (LD) with any of the VDR polymorphisms.13 The FokI F allele was found to influence immune cells behavior and may be associated with altered immune function, resulting in a more active immune system.14
Given the sparse data on VDR and CAP, we conducted this study to evaluate the association of the VDR gene Fok I polymorphism with susceptibility to CAP in young Egyptian children, and we also estimated serum 25D concentrations to assess its relation to Fok I polymorphism.
Methods
This was a prospective multicenter case-control study performed in Zagazig University, Ain-Shams and Cairo University hospitals from March 2016 through April 2018. The study protocol was approved by the ethical committees of Zagazig, Ain-Shams and Cairo Universities, Egypt and written informed consent from parents of each participant was provided in accordance with the Declaration of Helsinki.
All participants belonged to the same ethnic group: African Caucasian. A total of 300 children; who had CAP as diagnosed in the Pediatric Departments of the study hospitals, were recruited in this study. The study involved children aged from 6 months to 6 years. For the purpose of this study pneumonia was defined according to the previously published guidelines.15,16 Pneumonia was defined as community-acquired if children had no history of hospitalization during the two weeks prior to admission.17
Exclusion criteria
Children were excluded if they were recently hospitalized (4 weeks before admission), or had acute bronchiolitis or any alternative respiratory diagnosis, congenital heart disease (CHD), immunodeficiency, rickets, chronic hepatic or renal disease; and genetic or neurological disorders. Postoperative children and patients who received vitamin D or calcium supplementations during the past three months were also excluded.
Severity criteria
patients were further classified into three groups ‘mild, moderate or severe disease’ according to the British Thoracic Society guidelines.17 Severe sepsis (SS) was defined as systemic inflammatory response syndrome (SIRS) in association with multi-organ dysfunction.18 Acute Respiratory failure was defined as partial pressure of oxygen in arterial blood [PaO2] ≤ 50 mmHg in room air or [PaO2/FiO2] ratio ≤ 250 under oxygen administration and in the absence of cyanotic CHD.
The control population comprised three hundred healthy children aged 6 months to 6 years who attended the Pediatric Departments for preoperative evaluation for elective surgery (all without respiratory symptoms). To limit the effect of seasonal variation of vitamin D photosynthesis controls were also matched with cases by season at enrollment (winter or non-winter). All participants were subjected to detailed history taking and thorough clinical examination.
Blood sampling
Upon enrollment, a blood sample was obtained from each subject and divided into 2 ml blood collected into EDTA-containing tubes for genomic DNA isolation. Then, sera were separated and stored at −20 °C until processing.
Estimation of serum 25-hydroxy vitamin D concentration
Serum 25D was estimated using a commercial ELISA kit according to the manufacturer’s instructions (K2110, immune-diagnostic, Dutch Company, Holland). Vitamin D deficiency was considered as levels of 25D <20 ng/ml and severe deficiency for levels <10 ng/mL.19
Genomic DNA isolation
Genomic DNA from venous samples of all subjects was isolated using the Invisorb Spin Micro DNA Kit (Stratec Molecular GmbH, Berlin, Germany) according to the manufacturer’s protocol. DNA was stored at −20 °C till the time of use.
Genotyping of VDR Fok I gene polymorphisms
All subjects were genotyped for the Fok I (rs2228570) polymorphism located in exon 2 of the VDR gene by polymerase chain reaction– restriction fragment length polymorphism (PCR-RFLP) methodology. A 265 base-pair region was amplified by polymerase chain reaction (PCR), using the sense primer 5′- AGCTGGCCCTGGCACTGACTCTGCTCT-3′ and the antisense primer, 5′-ATGGAAACACCTTGCTTGCTTCTCCCTC-3′, as previously described.20
Statistical analysis
The VDR Fok I genotype and allele frequencies in patients with CAP and controls were tested for Hardy–Weinberg equilibrium. The Chi-square test was used to determine differences in the VDR Fok I allele frequencies and genotype distribution between patients and controls. Fisher’s exact test was used when the frequency was <5.The odds ratio (OR) and 95% Confidence Intervals (95% (CI)) were calculated for disease susceptibility in relation to the studied VDR Fok I gene variants. Multiple logistic regression analysis was performed to evaluate the independent effect of VDR Fok I genotypes on the clinical outcome of CAP. The Student's t-test and analysis of variance (ANOVA) test were used to compare numeric variables within groups. P value < 0.05 was considered to be statistically significant. All analyses were performed using SPSS for Windows, version 22.0 (IBM Corp., Armonk, NY).
Results
Of the 300 children with CAP enrolled in the study, 162 (54%) were males. The mean patient age was 2.3 years ranging from 6 to 72 months. The control group was well-matched for age, gender, ethnicity, and season at enrollment (all P > 0.05; Table 1).
The mean serum 25D level was lower in our patients compared to the control group (17.5 ± 4.4 ng/ml vs 36.7 ± 6.5 ng/ml; P < 0.01); Table 1.
Regarding severity, there were 102 (34%) patients identified as mild pneumonia, 117 (39%) as moderate and 81 (27%) as severe cases. Eighty-seven patients (29%) required admission to the ICU during their inpatient stay and 27 (9%) patients died. Eighty-one patients (27%) developed severe sepsis. Thirty-three cases (11%) were complicated with acute respiratory failure (Table 1).
Distribution of the VDR genotypes and alleles among studied subjects are shown in Table 2. The genotype frequencies of the VDR Fok I in patient and control groups were compatible with Hardy–Weinberg expectations.
The Fok I distributions were different between patients and control children. Homozygous FF was more common (21%) in patients than in healthy controls (6%). Children carrying the FF genotype had a 3.6 fold higher susceptibility to CAP (OR = 3.6; (95% CI: 1.9–6.7); P = 0.001). The occurrence of F allele was significantly more frequent in patients (44% vs. 30%; OR: 1.8; (95% CI: 1.4–2.3); P = 0.01); by contrast, a significant decrease in the frequency of the f allele was found in comparison to control children (56% vs. 70%; OR: 0.55; (95% CI: 0.4–0.7); P = 0.01); Table 2.
Thirty-two patients (51%) with the VDR FF genotype had severe pneumonia compared to (16 and 24%) in those carrying the ff or Ff genotypes, P < 0.01; Table 3). Severe sepsis was more frequent in the VDR FF homozygous subjects (52%) compared to (26%) in Ff heterozygous individuals and (12%) in ff genotypes. The VDR ff gene variant confers protection against sepsis (P = 0.001). The VDR Fok I genotypes were not associated with the risk of acute respiratory failure in CAP children (P = 0.375). Patients who required admission to the ICU during their inpatient stay had Fok I distributions as follow: the ff genotype (19%), Ff genotype (28%), and FF genotype (48%). The FF genotype carried the risk for ICU admission (P = 0.004). The ICU mortality was in significant association with the FF genotype (21%), meanwhile both Ff and ff gene variants were not (8 and 3%; respectively); (P = 0.001; Table 3).
Our logistic regression model showed a significant positive association between the VDR FF gene variant and susceptibility to sepsis, ICU admission, and hospital mortality as did the Fok I F allele (OR: 3.7; (95% CI: 1.4–10.2) for the FF genotype; P = 0.001) and (OR: 1.5; (95% CI: 1.3-5.8) for the F allele; P = 0.03.
About half of our CAP cases 159 (53%) were vitamin D deficient with 15% being severely deficient; meanwhile 141 (47%) cases had normal vitamin D levels. By contrast, 66 (22%) of the control children had vitamin D deficiency and 234 (78%) had normal vitamin D levels (P < 0.01; Fig. 1). Vitamin D deficient subjects had 4 folds higher susceptibility to CAP than did children with normal vitamin D level (OR = 4; (95% CI: 2.5–6.33), P < 0.01; Fig. 1).
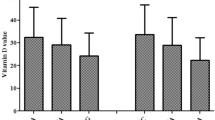
Patients carrying the VDR FF gene variant had lower serum 25D level (mean; 14.8 ± 3.6 ng/ml) than Ff genotype (20.6 ± 4.5 ng/ml) and the ff genotype (24.5 ± 3.7 ng/ml); P < 0.01.The mean serum 25D concentration was 15 ± 0.8 ng/ml for the Fok I F allele and 24.7 ± 1.4 ng/ml for the f allele; P < 0.01, Table 3.
Discussion
Childhood CAP is a common cause of mortality around the world.21 As early as 1975, a link was hypothesized between vitamin D and CAP.22 Experimental work confirmed that administration of vitamin D ameliorates pulmonary inflammatory responses while enhancing innate defense mechanisms against respiratory pathogens.23 In particular, the activation of the VDR by 1, 25D stimulate the endogenous synthesis of Cathelicidin which is cleaved to generate the active cationic peptide, LL-37. Cathelicidin is highly expressed at barrier sites including respiratory epithelium, thus provides a pivotal first line defense for the innate immune system.24 In addition, vitamin D regulates phagocytosis dependent and antibody dependent macrophages which protect from respiratory infections.25 Vitamin D was also reported as a growth factor for alveolar cells type-II, and local conversion of 25D to 1, 25D was once considered as a key modulator of epithelial proliferation in the context of lung development and repair.26
Because of potential immune-modulatory activity of vitamin D and its effects on pulmonary cell biology, the VDR gene polymorphisms may affect individuals’ susceptibility, illness severity, and the clinical outcome of CAP.
In this study, the VDR Fok I genotypic distribution was different between studied groups. The FF genotype and F allele were more frequent in patients than in control group. A child with the FF genotype had 3.6-increased odds for CAP risk, thus revealing that our cases were more susceptible to pneumonia. By contrast, the Fok I f allele showed a significant negative association with CAP risk suggesting that f allele confers protection against CAP.
SNPs in the VDR gene have been reported to be associated with a variety of physiological and pathological phenotypes. Recent studies discovered an association between some VDR gene variants with host susceptibility to pulmonary tuberculosis,27 symptomatic pertussis28 and RSV bronchiolitis in children.29 To date, few reports concerned the association of the VDR gene SNPs with susceptibility to CAP.30,31 Li et al. studied the VDR Fok I gene SNP (rs2239185) on genomic DNAs of 91 Chinese Han population, compared to 94 ethnicity-matched controls. The authors demonstrated that the TT genotype of rs2239185 in the VDR gene may be a genetic risk factor for CAP, and the T allele at the same position may be associated with CAP susceptibility and severity.30 Roth et al.31 investigated the role of VDR gene SNPs in acute lower respiratory tract infections among Canadian children. By contrast, they reported that the odds of LRTI for children with the Fok I ff gene variant were increased relative to those with the Fok I FF genotype. They concluded that a function altering SNP in the VDR gene was associated with more risk for LRTI.
The inconsistent findings between our study and previously reported data are likely related to different ethnicity or to genetic and environmental interplay.
A high prevalence of vitamin D deficiency was demonstrated in pediatric and adolescent populations across the globe. It has been postulated that vitamin D deficiency may contribute to the epidemic of LRTI, including CAP, among Egyptian, Ethiopian, and Japanese cohorts.9,32,33
Of note, vitamin D deficiency was detected in about half (53%) of CAP patients with (15%) being severely deficient; although the study population reside in Delta Egypt with plenty of sunny weather. In line with our expectations, the mean serum 25D level was significantly decreased in children with CAP compared to the control group. Moreover, children with the Fok I FF genotype had significantly lower serum 25D concentrations relative to the Ff and ff gene variants. This finding confirms the results of recently published reports.34
1, 25D regulates its own serum concentration and its precursor 25D by a negative feedback loop via the VDRs. Unlike BsmI, ApaI and TaqI polymorphisms, FokI polymorphism gives rise to VDR proteins of different lengths. The long allelic variant, i.e., f-allele has less biological activity than the shorter F-VDR,14 therefore, allow more synthesis of 25D, which could explain the observed higher concentrations of vitamin D in children with the FokI ff gene variant.
Our findings support the possibility that the VDR gene SNPs may contribute to inter-individual variations in the course of illness and clinical outcome of CAP.
Among studied children with CAP, the presence of the VDR allele F or the FF gene variant; being associated with lower serum 25D levels; constitute risk factor for developing severe sepsis, ICU admission and mortality. Our results confirm and extend the previous findings of Das et al.35 who suggested that the VDR polymorphisms can be potentially used as genetic markers for assessing sepsis risk in Indian population.
In experimental models of sepsis, 1, 25D administration has been shown to modulate systemic inflammatory cytokine response and was associated with improved coagulation parameters in sepsis related disseminated intravascular coagulation.36 In vitro study by Liu et al. confirmed that 1, 25D treatment of cultured macrophages infected with M. tuberculosis resulted in enhanced expression of cathelicidin, and improved killing of the microorganisms.37 Cathelicidin has a broad antimicrobial spectrum against gram negative and positive bacteria, fungi and mycobacteria.24
Based on a review of the epidemiology of sepsis, it was hypothesized that vitamin D insufficiency is a risk factor for sepsis worldwide. That hypothesis was quickly supported by Jeng et al.,38 who found that those admitted to ICU with or without sepsis had much lower serum vitamin D levels than others in the community. Inamo et al.33 suggested a correlation between severe 25D deficiencies and the need of supplementary oxygen and ventilator support in children with acute LRTI.
Moreover, Lee et al.39 reported increased hospital mortality in the critically ill with vitamin D deficiency that may be explained by immune and endothelial cell dysfunction. In particular, endothelial cell dysfunction has been supposed to be a potential cause of multiple organ dysfunction syndrome (MODS).40 It is possible that vitamin D deficiency amplifies the impaired immune regulation and metabolic derangement observed in sepsis, which may lead to worse clinical outcome than would be experienced in children with normal vitamin D status.
At the molecular level, vitamin D suppresses the amassing of mRNA for interleukin (IL)-12, GM-CSF, and interferon –γ while IL-4, IL-10, and transforming growth factor β (TGF-β) production is enhanced, resulting in inhibition of the overall helper T cell type 1 response (Th1) and further shifts the cytokine profile toward Th2 dominance. The VDR and its ligand, 1, 25D, has been shown to enhance the differentiation of naive CD4 + T lymphocytes toward a helper T cell type 2.41 Hence, Vitamin D exerts both anti-microbial and anti-inflammatory effects against infectious pathogens.8 In Vitamin D deficiency or even insufficiency, there is decreased expression of the VDRs resulting in impaired clearance of microbes and uncontrolled pulmonary inflammation that leads to progressive lung damage with impaired oxygenation.42
To our knowledge, ours is the first such study to evaluate the association between the VDR Fok I polymorphism and the susceptibility to CAP in Egyptian children.
Since the small sample size was one of our limitations; it may be necessary to adopt a genome-wide association studies; particularly in our developing countries.
A lack of detailed dietary intake or sun exposure data among children with CAP was another limitation in our study. We have studied only one SNP in the VDR gene which might represent LD with yet-to-be-identified other VDR gene markers. As vitamin D measurements and the CAP diagnosis were concurrent, cause and effect assumptions are difficult. Other VDR polymorphisms (i.e., BsmI, ApaI, and TaqI) should be genotyped concurrently with evaluation of vitamin D status in children with CAP to validate these findings on different ethnic populations.
Conclusion
The VDR gene Fok I (rs2228570) polymorphism confers susceptibility to CAP in Egyptian children.
Finally, Vitamin D deficiency may be a modifiable risk factor for morbidity in CAP and represents a promising target for intervention taking into account the VDR polymorphisms.
References
Wardlaw, T., Salama, P., Johansson, E. W. & Mason, E. Pneumonia: the leading killer of children. Lancet 368, 1048–1050 (2006).
Solé- Violán, J. et al. Genetic variability in the severity and outcome of community-acquired pneumonia. Respir. Med. 104, 440–447 (2010).
Patwari, P. P. et al. Interleukin-1 receptor antagonist intron 2 variable number of tandem repeats polymorphism and respiratory failure in children with community-acquired pneumonia. Pediatr. Crit. Care. Med. 9, 553–559 (2008).
Pike, J. W. & Meyer, M. B. The vitamin D receptor: new paradigms for the regulation of gene expression by 1,25-dihydroxyvitamin D(3). Endocrinol. Metab. Clin. North. Am. 39, 255–269 (2010).
Underwood, M. A. & Bevins, C. L. Defensin-barbed innate immunity: clinical associations in the pediatric population. Pediatrics 125, 1237–1247 (2010).
Hossein-nezhad, A. & Holick, M. F. Vitamin D for health: a global perspective. Mayo Clin. Proc. 88, 720–755 (2013).
Liu, P. T., Stenger, S., Tang, D. H. & Modlin, R. L. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J. Immunol. 179, 2060–2063 (2007).
Khoo, A. L. et al. Translating the role of vitamin D3 in infectious diseases. Crit. Rev. Microbiol. 38, 122–135 (2012).
Muhe, L., Lulseged, S., Mason, K. E. & Simoes, E. A. Case-control study of the role of nutritional rickets in the risk of developing pneumonia in Ethiopian children. Lancet 349, 1801–1804 (1997).
Banajeh, S. M. Nutritional rickets and vitamin D deficiency–association with the outcomes of childhood very severe pneumonia: a prospective cohort study. Pediatr. Pulmonol. 44, 1207–1215 (2009).
Manaseki-Holland, S. et al. Effects of vitamin D supplementation to children diagnosed with pneumonia in Kabul: a randomised controlled trial. Trop. Med. Int. Health 15, 1148–1155 (2010).
O Neill, V., Asani, F. F., Jeffery, T. J., Saccone, D. S. & Bornman, L. Vitamin D receptor gene expression and function in a South African population: ethnicity, vitamin D and FokI. PLoS ONE 8, e67663 (2013).
Nejentsev, S. et al. Comparative high-resolution analysis of linkage disequilibrium and tag single nucleotide polymorphisms between populations in the vitamin D receptor gene. Hum. Mol. Genet. 13, 1633–1639 (2004).
van Etten, E. et al. The vitamin D receptor gene FokI polymorphism: functional impact on the immune system. Eur. J. Immunol. 37, 395–405 (2007).
Neuman, M. I., Monuteaux, M. C., Scully, K. J. & Bachur, R. G. Prediction of pneumonia in a pediatric emergency department. Pediatrics 128, 246–253 (2011).
World Health Organization: Standardization of interpretation of chest radiographs for the diagnosis of pneumonia in children. In WHO/V&B/01.35. Pneumonia Vaccine Trials Investigator's Group. Geneva: World Health Organization; 2001:32. http://www.who.int/iris/handle/10665/66956
British Thoracic Society of Standards of Care Committee. BTS Guidelines for the management of community acquired pneumonia in childhood. Thorax 57, 1i–24i (2011).
Goldstein, B., Giroir, B. & Randolph, A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr. Crit. Care. Med. 6, 2–8 (2005).
Holick, M. F. Vitamin D deficiency. N. Engl. J. Med. 357, 266–281 (2007).
Mory, D. B. et al. Prevalence of vitamin D receptor gene polymorphisms FokI and BsmI in Brazilian individuals with type 1 diabetes and their relation to beta-cell autoimmunity and to remaining beta-cell function. Hum. Immunol. 70, 447–451 (2009).
Harris, M. et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax 66(Suppl 2), ii1–ii23 (2011).
Salimpour, R. Rickets in Tehran. Study of 200 cases. Arch. Dis. Child. 50, 63–66 (1975).
Hughes, D. A. & Norton, R. Vitamin D and respiratory health. Clin. Exp. Immunol. 158, 20–25 (2009).
Dürr, U. H., Sudheendra, U. S. & Ramamoorthy, A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim. Biophys. Acta 1758, 1408–1425 (2006).
Raloff, J. The antibiotic vitamin: deficiency in vitamin D may predispose people to infection. Sci. News 170, 312–317 (2006).
Edelson, J. D., Chan, S., Jassal, D., Post, M. & Tanswell, A. K. Vitamin D stimulates DNA synthesis in alveolar type-II cells. Biochim. Biophys. Acta 1221, 159–166 (1994).
Gao, L., Tao, Y., Zhang, L. & Jin, Q. Vitamin D receptor genetic polymorphisms and tuberculosis: updated systematic review and meta-analysis. Int. J. Tuberc. Lung. Dis. 14, 15–23 (2010).
Han, W. G. et al. Association of vitamin D receptor polymorphism with susceptibility to symptomatic pertussis. PLoS ONE 11, e0149576 (2016).
Kresfelder, T. L., Janssen, R., Bont, L., Pretorius, M. & Venter, M. Confirmation of an association between single nucleotide polymorphisms in the VDR gene with respiratory syncytial virus related disease in South African children. J. Med. Virol. 83, 1834–1840 (2011).
Li, W. et al. Polymorphism rs2239185 in vitamin D receptor gene is associated with severe community-acquired pneumonia of children in Chinese Han population: a case–control study. Eur. J. Pediatr. 174, 621–629 (2015).
Roth, D. E., Jones, A. D., Prosser, C., Robinson, J. L. & Vohra, S. Vitamin D receptor polymorphism and the risk of acute lower respiratory tract infection in early childhood. J. Infect. Dis. 197, 676–680 (2008).
El Basha, N., Mohsen, M., Kamal, M. & Mehaney, D. Association of vitamin D deficiency with severe pneumonia in hospitalized children under 5 years. Comp. Clin. Pathol. 23, 1247–1252 (2014).
Inamo, Y. et al. Serum vitamin D concentrations and associated severity of acute lower respiratory tract infections in Japanese hospitalized children. Pediatr. Int. 53, 199–201 (2011).
Monticielo, O. A. et al. The role of BsmI and FokI vitamin D receptor gene polymorphisms and serum 25-hydroxyvitamin D in Brazilian patients with systemic lupus erythematosus. Lupus 21, 43–52 (2012).
Das, B., Patra, S., Behera, C. & Suar, M. Genotyping of vitamin D receptor gene polymorphisms using mismatched amplification mutation assay in neonatal sepsis patients of Odisha, eastern India. Infect. Genet. Evol. 45, 40–47 (2016).
Møller, S., Laigaard, F., Olgaard, K. & Hemmingsen, C. Effect of 1,25-dihydroxy-vitamin D3 in experimental sepsis. Int. J. Med. Sci. 4, 190–195 (2007).
Liu, P. T. et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311, 1770–1773 (2006).
Jeng, L. et al. Alterations in vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. J. Transl. Med. 7, 28 (2009).
Lee, P., Nair, P., Eisman, J. A. & Center, J. R. Vitamin D deficiency in the intensive care unit: an invisible accomplice to morbidity and mortality? Intensive Care Med. 35, 2028–2032 (2009).
Aird, W. C. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood 101, 3765–3777 (2003).
Kamen, D. L. & Tangpricha, V. Vitamin D and molecular actions on the immune system: modulation of innate and autoimmunity. J. Mol. Med. 88, 441–450 (2010).
Yorita, K. L. et al. Severe bronchiolitis and respiratory syncytial virus among young children in Hawaii. Pediatr. Infect. Dis. J. 26, 1081–1088 (2007).
Acknowledgements
We thank the staff of Pediatric Pulmonology and Outpatient Clinics in Zagazig University, Ain-Shams and Cairo University hospitals for their collaboration in sampling as well as our patients who participated in the study.
Author contributions
H.A.Z. submitted the manuscript. M.A.A. designed the study. A.M.K. collected clinical data and coordinated the sample collection (Zagazig University). N.M.A. collected clinical data and coordinated the sample collection (Ain-Shams University). M.M.S. collected clinical data and coordinated the sample collection (Cairo University). M.S.H. and H.A.A.E. performed the statistical analysis. M.A.N. and A.A.S. helped to draft the manuscript. A.M.S., A.A.M., and M.E.H. wrote the manuscript. A.A.A., M.T.Z., and S.S.A.E. critically revised the final version. A.M.A., R.M.N., and G.M.A. performed laboratory analysis and genotyping. All authors read and approved all the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abouzeid, H., Abdelaal, N.M., Abdou, M.A. et al. Association of vitamin D receptor gene FokI polymorphism and susceptibility to CAP in Egyptian children: a multicenter study. Pediatr Res 84, 639–644 (2018). https://doi.org/10.1038/s41390-018-0149-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-018-0149-y
This article is cited by
-
Increased susceptibility to complicated pneumonia among egyptian children with FokI (rs2228570), not TaqI (rs731236), vitamin D receptor gene polymorphism in association with vitamin D deficiency: a case-control study
BMC Pediatrics (2023)
-
Vitamin D deficiency and vitamin D receptor FokI polymorphism as risk factors for COVID-19
Pediatric Research (2023)
-
Vitamin D receptor polymorphisms and vitamin D insufficiency are not associated with sepsis in critically ill children: a case-control study
Egyptian Pediatric Association Gazette (2022)
-
Vitamin D receptor, vitamin D binding protein and CYP27B1 single nucleotide polymorphisms and susceptibility to viral infections in infants
Scientific Reports (2021)