Abstract
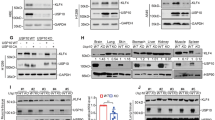
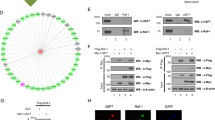
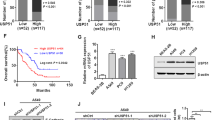
Protein ubiquitination is a common post-translational modification and a critical mechanism for regulating protein stability. This study aimed to explore the role and potential molecular mechanism of ubiquitin-specific peptidase 38 (USP38) in the progression of lung adenocarcinoma (LUAD). USP38 expression was significantly higher in patients with LUAD than in their counterparts, and higher USP38 expression was closely associated with a worse prognosis. USP38 silencing suppresses the proliferation of LUAD cells in vitro and impedes the tumorigenic activity of cells in xenograft mouse models in vivo. Further, we found that USP38 affected the protein stability of transcription factor Krüppel-like factors 5 (KLF5) by inhibiting its degradation. Subsequent mechanistic investigations showed that the N-terminal of USP38 (residues 1-400aa) interacted with residues 1-200aa of KLF5, thereby stabilizing the KLF5 protein by deubiquitination. Moreover, we found that PIAS1-mediated SUMOylation of USP38 was promoted, whereas SENP2-mediated de-SUMOylation of USP38 suppressed the deubiquitination effects of USP38 on KLF5. Additionally, our results demonstrated that KLF5 overexpression restored the suppression of the malignant properties of LUAD cells by USP38 knockdown. SUMOylation of USP38 enhances the deubiquitination and stability of KLF5, thereby augmenting the malignant progression of LUAD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All relevant data are available from the authors upon request.
References
Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446–54.
Erratum: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2020;70:313.
Reck M, Heigener DF, Mok T, Soria J-C, Rabe KF. Management of non-small-cell lung cancer: recent developments. Lancet. 2013;382:709–19.
Magiera MM, Singh P, Janke C. SnapShot: functions of tubulin posttranslational modifications. Cell. 2018;173:1552–1552.e1.
Hochstrasser M. Origin and function of ubiquitin-like proteins. Nature. 2009;458:422–9.
Gallo LH, Ko J, Donoghue DJ. The importance of regulatory ubiquitination in cancer and metastasis. Cell Cycle. 2017;16:634–48.
Pineda CT, Ramanathan S, Fon Tacer K, Weon JL, Potts MB, Ou Y-H, et al. Degradation of AMPK by a cancer-specific ubiquitin ligase. Cell. 2015;160:715–28.
Parvatiyar K, Harhaj EW. Cell signaling. Anchors away for ubiquitin chains. Science. 2010;328:1244–5.
Komander D, Clague MJ, Urbé S. Breaking the chains: structure and function of the deubiquitinases. Nat Rev Mol Cell Biol. 2009;10:550–63.
Abdul Rehman SA, Kristariyanto YA, Choi S-Y, Nkosi PJ, Weidlich S, Labib K, et al. MINDY-1 Is a member of an evolutionarily conserved and structurally distinct new family of deubiquitinating enzymes. Mol Cell. 2016;63:146–55.
Nijman SMB, Luna-Vargas MPA, Velds A, Brummelkamp TR, Dirac AMG, Sixma TK, et al. A genomic and functional inventory of deubiquitinating enzymes. Cell. 2005;123:773–86.
Tang J, Luo Y, Xiao L. USP26 promotes anaplastic thyroid cancer progression by stabilizing TAZ. Cell Death Dis. 2022;13:326.
Sheng Y, Saridakis V, Sarkari F, Duan S, Wu T, Arrowsmith CH, et al. Molecular recognition of p53 and MDM2 by USP7/HAUSP. Nat Struct Mol Biol. 2006;13:285–91.
Hu M, Gu L, Li M, Jeffrey PD, Gu W, Shi Y. Structural basis of competitive recognition of p53 and MDM2 by HAUSP/USP7: implications for the regulation of the p53-MDM2 pathway. PLoS Biol. 2006;4:e27.
Tavana O, Li D, Dai C, Lopez G, Banerjee D, Kon N, et al. HAUSP deubiquitinates and stabilizes N-Myc in neuroblastoma. Nat Med. 2016;22:1180–6.
Wu H-T, Kuo Y-C, Hung J-J, Huang C-H, Chen W-Y, Chou T-Y, et al. K63-polyubiquitinated HAUSP deubiquitinates HIF-1α and dictates H3K56 acetylation promoting hypoxia-induced tumour progression. Nat Commun. 2016;7:13644.
Wang Q, Ma S, Song N, Li X, Liu L, Yang S, et al. Stabilization of histone demethylase PHF8 by USP7 promotes breast carcinogenesis. J Clin Investig. 2016;126:2205–20.
Yang Y, Yang C, Li T, Yu S, Gan T, Hu J, et al. The deubiquitinase USP38 promotes NHEJ repair through regulation of HDAC1 activity and regulates cancer cell response to genotoxic insults. Cancer Res. 2020;80:719–31.
Chen S, Yun F, Yao Y, Cao M, Zhang Y, Wang J, et al. USP38 critically promotes asthmatic pathogenesis by stabilizing JunB protein. J Exp Med. 2018;215:2850–67.
Lin M, Zhao Z, Yang Z, Meng Q, Tan P, Xie W, et al. USP38 inhibits Type I interferon signaling by editing TBK1 ubiquitination through NLRP4 signalosome. Mol Cell. 2016;64:267–81.
Flotho A, Melchior F. Sumoylation: a regulatory protein modification in health and disease. Annu Rev Biochem. 2013;82:357–85.
Li Y, Varejão N, Reverter D. Structural basis for the SUMO protease activity of the atypical ubiquitin-specific protease USPL1. Nat Commun. 2022;13:1819.
Zhen Y, Knobel PA, Stracker TH, Reverter D. Regulation of USP28 deubiquitinating activity by SUMO conjugation. J Biol Chem. 2014;289:34838–50.
Lopitz-Otsoa F, Delgado TC, Lachiondo-Ortega S, Azkargorta M, Elortza F, Rodríguez MS. et al. SUMO-binding entities (SUBEs) as tools for the enrichment, isolation, identification, and characterization of the SUMO proteome in liver cancer. J Vis Exp. 2019;153:60098.
Yeh ET. SUMOylation and De-SUMOylation: wrestling with life’s processes. J Biol Chem. 2009;284:8223–7.
Salas-Lloret D, González-Prieto R. Insights in post-translational modifications: ubiquitin and SUMO. Int J Mol Sci. 2022;23:3281.
Praefcke GJK, Hofmann K, Dohmen RJ. SUMO playing tag with ubiquitin. Trends Biochem Sci. 2012;37:23–31.
Wang Y, Li Q, Hu D, Gao D, Wang W, Wu K, et al. USP38 Inhibits Zika Virus Infection by Removing Envelope Protein Ubiquitination. Viruses. 2021;13:2029.
Zhan W, Liao X, Liu J, Tian T, Yu L, Li R. USP38 regulates the stemness and chemoresistance of human colorectal cancer via regulation of HDAC3. Oncogenesis. 2020;9:48.
Zheng Z, Shang Y, Xu R, Yan X, Wang X, Cai J, et al. Ubiquitin specific peptidase 38 promotes the progression of gastric cancer through upregulation of fatty acid synthase. Am J Cancer Res. 2022;12:2686–96.
Lyu Y, Guan Y, Deliu L, Humphrey E, Frontera JK, Yang YJ, et al. KLF5 governs sphingolipid metabolism and barrier function of the skin. Genes Dev. 2022;36:822–42.
Li X, Liu X, Xu Y, Liu J, Xie M, Ni W, et al. KLF5 promotes hypoxia-induced survival and inhibits apoptosis in non-small cell lung cancer cells via HIF-1α. Int J Oncol. 2014;45:1507–14.
Zhao C, Li Y, Qiu W, He F, Zhang W, Zhao D, et al. C5a induces A549 cell proliferation of non-small cell lung cancer via GDF15 gene activation mediated by GCN5-dependent KLF5 acetylation. Oncogene. 2018;37:4821–37.
Shi J, Yang C, An J, Hao D, Liu C, Liu J, et al. KLF5-induced BBOX1-AS1 contributes to cell malignant phenotypes in non-small cell lung cancer via sponging miR-27a-5p to up-regulate MELK and activate FAK signaling pathway. J Exp Clin Cancer Res. 2021;40:148.
Zhang T, Wang B, Su F, Gu B, Xiang L, Gao L, et al. TCF7L2 promotes anoikis resistance and metastasis of gastric cancer by transcriptionally activating PLAUR. Int J Biol Sci. 2022;18:4560–77.
Acknowledgements
This work was supported by funding from National Natural Science Foundation of China (81802269 and 82260518); Medical Innovation and Development Project of Lanzhou University (lzuyxcx-2022-183); Construction Project of Clinical Medical Research Center from Gansu Provincial Department of Science and Technology (21JR7RA390); Key Research and Development Plan from Gansu Provincial Department of Science and Technology (22YF7FA086); Unite Pesearch Foundation of Gansu Province (23JRRA1497); Natural Science Foundation of Gansu Province (20JR5RA352 and 22JR5RA918); Youth Science and Technology Talent Innovation Project of Lanzhou City (2023-QN-14); Scientific and Technological Development Guiding Plan Project of Lanzhou City (2020-ZD-74).
Author information
Authors and Affiliations
Contributions
T. Zhang: Data curation, formal analysis, validation, visualization, methodology, writing–original draft, writing–review and editing. F. Su: Formal analysis, validation, visualization, methodology. B. Wang: Data curation, software, formal analysis, visualization, methodology. Y. Lu: Methodology. H. Su: Data curation, formal analysis. R. Ling: Methodology. P. Yue: Methodology. H. Dai: Methodology. T. Yang: Methodology. J. Yang: Funding acquisition, methodology. R. Chen: Methodology. R. Wu: Methodology. K. Zhu: Methodology. R. Chen: Conceptualization, supervision, project administration. D. Zhao: Conceptualization, supervision, funding acquisition, writing–original draft, project administration, writing–review and editing. X. Hou: Conceptualization, supervision, funding acquisition, writing–original draft, project administration, writing– review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, T., Su, F., Wang, B. et al. Ubiquitin specific peptidase 38 epigenetically regulates KLF transcription factor 5 to augment malignant progression of lung adenocarcinoma. Oncogene 43, 1190–1202 (2024). https://doi.org/10.1038/s41388-024-02985-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-024-02985-7