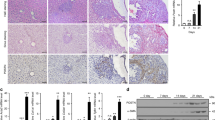
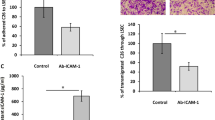
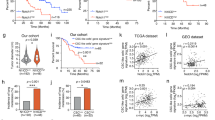
Abstract
Metastasis causes most cancer-related deaths, and the role and mechanism of periostin (POSTN) in the metastasis of hepatocellular carcinoma (HCC) remain undiscovered. In this study, DEN and HTVi HCC models were performed in hepatic-specific Postn ablation and Postn knock-in mouse to reveal the role of POSTN in HCC metastasis. Furthermore, POSTN was positively correlated with circulating EPCs level and promoted EPC mobilization and tumour infiltration. POSTN also mediated the crosstalk between HCC and EPCs, which promoted metastasis ability and upregulated CD36 expression in HCC through indirect crosstalk. Chemokine arrays further revealed that hepatic-derived POSTN induced elevated CCL2 expression and secretion in EPCs, and CCL2 promoted prometastatic traits in HCC. Mechanistic studies showed that POSTN upregulated CCL2 expression in EPCs via the αvβ3/ILK/NF-κB pathway. CCL2 further induced CD36 expression via the CCR2/STAT3 pathway by directly binding to the promoter region of CD36. Finally, CD36 was verified to have a prometastatic role in vitro and to be correlated with POSTN expression, metastasis and recurrence in HCC in clinical samples. Our findings revealed that crosstalk between HCC and EPCs is mediated by periostin/CCL2/CD36 signalling which promotes HCC metastasis and emphasizes a potential therapeutic strategy for preventing HCC metastasis.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All data and materials supporting the findings of this work are available from its supplementary information files and from the corresponding author upon reasonable request.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the global burden of disease study 2015. JAMA Oncol. 2017;3:1683–91.
Llovet J, De Baere T, Kulik L, Haber P, Greten T, Meyer T, et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18:293–313.
Wasik A, Ratajczak-Wielgomas K, Badzinski A, Dziegiel P, Podhorska-Okolow M. The role of periostin in angiogenesis and lymphangiogenesis in tumors. Cancers. 2022;14:4225.
Chen G, Wang Y, Zhao X, Xie XZ, Zhao JG, Deng T, et al. A positive feedback loop between Periostin and TGFβ1 induces and maintains the stemness of hepatocellular carcinoma cells via AP-2α activation. J Exp Clin Cancer Res. 2021;40:218.
Chen G, Nakamura I, Dhanasekaran R, Iguchi E, Tolosa EJ, Romecin PA, et al. Transcriptional induction of periostin by a sulfatase 2-TGFβ1-SMAD signaling axis mediates tumor angiogenesis in hepatocellular carcinoma. Cancer Res. 2017;77:632–45.
Gillot L, Lebeau A, Baudin L, Pottier C, Louis T, Durré T, et al. Periostin in lymph node pre-metastatic niches governs lymphatic endothelial cell functions and metastatic colonization. Cell Mol Life Sci. 2022;79:295.
Xiao H, Zhang Y, Li Z, Liu B, Cui D, Liu F, et al. Periostin deficiency reduces diethylnitrosamine-induced liver cancer in mice by decreasing hepatic stellate cell activation and cancer cell proliferation. J Pathol. 2021;255:212–23.
Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319:195–8.
Shih Y, Wang M, Zhou J, Peng H, Lee D, Chiu J. Endothelial progenitors promote hepatocarcinoma intrahepatic metastasis through monocyte chemotactic protein-1 induction of microRNA-21. Gut. 2015;64:1132–47.
Viallard C, Larrivée B. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis. 2017;20:409–26.
Yu D, Sun X, Qiu Y, Zhou J, Wu Y, Zhuang L, et al. Identification and clinical significance of mobilized endothelial progenitor cells in tumor vasculogenesis of hepatocellular carcinoma. Clin Cancer Res. 2007;13:3814–24.
Ho JW, Pang RW, Lau C, Sun CK, Yu WC, Fan ST, et al. Significance of circulating endothelial progenitor cells in hepatocellular carcinoma. Hepatol. 2006;44:836–43.
Massagué J, Ganesh K. Metastasis-initiating cells and ecosystems. Cancer Discov. 2021;11:971–94.
Castro-Giner F, Aceto N. Tracking cancer progression: from circulating tumor cells to metastasis. Genome Med. 2020;12:31.
Ganesh K, Massagué J. Targeting metastatic cancer. Nat Med. 2021;27:34–44.
Bian X, Liu R, Meng Y, Xing D, Xu D, Lu Z. Lipid metabolism and cancer. J Exp Med. 2021;218:e20201606.
Su X, Abumrad NA. Cellular fatty acid uptake: a pathway under construction. Trends Endocrinol Metab. 2009;20:72–77.
Pascual G, Avgustinova A, Mejetta S, Martín M, Castellanos A, Attolini CS, et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature. 2017;541:41–45.
Ladanyi A, Mukherjee A, Kenny HA, Johnson A, Mitra AK, Sundaresan S, et al. Adipocyte-induced CD36 expression drives ovarian cancer progression and metastasis. Oncogene. 2018;37:2285–301.
Pfeiler S, Thakur M, Grünauer P, Megens RTA, Joshi U, Coletti R, et al. CD36-triggered cell invasion and persistent tissue colonization by tumor microvesicles during metastasis. FASEB J. 2019;33:1860–72.
Luo X, Zheng E, Wei L, Zeng H, Qin H, Zhang X, et al. The fatty acid receptor CD36 promotes HCC progression through activating Src/PI3K/AKT axis-dependent aerobic glycolysis. Cell Death Dis. 2021;12:328.
Duan F, Wu H, Jia D, Wu W, Ren S, Wang L, et al. O-GlcNAcylation of RACK1 promotes hepatocellular carcinogenesis. J Hepatol. 2018;68:1191–202.
Robertson CL, Srivastava J, Siddiq A, Gredler R, Emdad L, Rajasekaran D, et al. Genetic deletion of AEG-1 prevents hepatocarcinogenesis. Cancer Res. 2014;74:6184–93.
García-Irigoyen O, Latasa MU, Carotti S, Uriarte I, Elizalde M, Urtasun R, et al. Matrix metalloproteinase 10 contributes to hepatocarcinogenesis in a novel crosstalk with the stromal derived factor 1/C-X-C chemokine receptor 4 axis. Hepatol. 2015;62:166–78.
Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Hicklin DJ, et al. Halting the interaction between vascular endothelial growth factor and its receptors attenuates liver carcinogenesis in mice. Hepatol. 2004;39:1517–24.
Lei MML, Leung CON, Lau EYT, Leung RWH, Ma VWS, Tong M, et al. SCYL3, as a novel binding partner and regulator of ROCK2, promotes hepatocellular carcinoma progression. JHEP Rep. 2023;5:100604.
Wang Y, Yu H, Shan Y, Tao C, Wu F, Yu Z, et al. EphA1 activation promotes the homing of endothelial progenitor cells to hepatocellular carcinoma for tumor neovascularization through the SDF-1/CXCR4 signaling pathway. J Exp Clin Cancer Res. 2016;35:65.
Suresh A, Biswas A, Perumal S, Khurana S. Periostin and integrin signaling in stem cell regulation. Adv Exp Med Biol. 2019;1132:163–76.
Ma Z, Zhao X, Deng M, Huang Z, Wang J, Wu Y, et al. Bone marrow mesenchymal stromal cell-derived periostin promotes B-ALL progression by modulating CCL2 in Leukemia Cells. Cell Rep. 2019;26:1533–43.e4.
Lv Y, Wang W, Jia WD, Sun QK, Li JS, Ma JL, et al. High-level expression of periostin is closely related to metastatic potential and poor prognosis of hepatocellular carcinoma. Med Oncol. 2013;30:385.
Wang Z, An J, Zhu D, Chen H, Lin A, Kang J, et al. Periostin: an emerging activator of multiple signaling pathways. J Cell Commun Signal. 2022;16:515–30.
Zhang R, Lin XH, Ma M, Chen J, Chen J, Gao DM, et al. Periostin involved in the activated hepatic stellate cells-induced progression of residual hepatocellular carcinoma after sublethal heat treatment: its role and potential for therapeutic inhibition. J Transl Med. 2018;16:302.
Krishna Priya S, Nagare RP, Sneha VS, Sidhanth C, Bindhya S, Manasa P, et al. Tumour angiogenesis-origin of blood vessels. Int J Cancer. 2016;139:729–35.
Ling CC, Ng KT, Shao Y, Geng W, Xiao JW, Liu H, et al. Post-transplant endothelial progenitor cell mobilization via CXCL10/CXCR3 signaling promotes liver tumor growth. J Hepatol. 2014;60:103–9.
Rafii S, Lyden D, Benezra R, Hattori K, Heissig B. Vascular and haematopoietic stem cells: novel targets for anti-angiogenesis therapy? Nat Rev Cancer. 2002;2:826–35.
Rafii S, Meeus S, Dias S, Hattori K, Heissig B, Shmelkov S, et al. Contribution of marrow-derived progenitors to vascular and cardiac regeneration. Semin Cell Dev Biol. 2002;13:61–7.
Wang G, Wen B, Deng Z, Zhang Y, Kolesnichenko O, Ustiyan V, et al. Endothelial progenitor cells stimulate neonatal lung angiogenesis through FOXF1-mediated activation of BMP9/ACVRL1 signaling. Nat Commun. 2022;13:2080.
Zarychta E, Ruszkowska-Ciastek B, Bielawski K, Rhone P. Stromal cell-derived factor 1α (SDF-1α) in invasive breast cancer: associations with vasculo-angiogenic factors and prognostic significance. Cancers. 2021;13:1952.
Vanharanta S, Massagué J. Origins of metastatic traits. Cancer Cell. 2013;24:410–21.
Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell. 2017;168:670–91.
Jiang M, Wu N, Xu B, Chu Y, Li X, Su S, et al. Fatty acid-induced CD36 expression via O-GlcNAcylation drives gastric cancer metastasis. Theranostics. 2019;9:5359–73.
Nath A, Li I, Roberts LR, Chan C. Elevated free fatty acid uptake via CD36 promotes epithelial-mesenchymal transition in hepatocellular carcinoma. Sci Rep. 2015;5:14752.
Martin-Perez M, Urdiroz-Urricelqui U, Bigas C, Benitah S. The role of lipids in cancer progression and metastasis. Cell Metab. 2022;34:1675–99.
Aoki T, Kinoshita J, Munesue S, Hamabe-Horiike T, Yamaguchi T, Nakamura Y, et al. Hypoxia-induced CD36 expression in gastric cancer cells promotes peritoneal metastasis via fatty acid uptake. Ann Surg Oncol. 2023;30:3125–36.
Yang P, Qin H, Li Y, Xiao A, Zheng E, Zeng H, et al. CD36-mediated metabolic crosstalk between tumor cells and macrophages affects liver metastasis. Nat Commun. 2022;13:5782.
Lim SY, Yuzhalin AE, Gordon-Weeks AN, Muschel RJ. Targeting the CCL2-CCR2 signaling axis in cancer metastasis. Oncotarget. 2016;7:28697–710.
Yoshimura T. The chemokine MCP-1 (CCL2) in the host interaction with cancer: a foe or ally? Cell Mol Immunol. 2018;15:335–45.
Wang WW, Ang SF, Kumar R, Heah C, Utama A, Tania NP, et al. Identification of serum monocyte chemoattractant protein-1 and prolactin as potential tumor markers in hepatocellular carcinoma. PloS One. 2013;8:e68904.
Li X, Yao W, Yuan Y, Chen P, Li B, Li J, et al. Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut. 2017;66:157–67.
Wang T, Fahrmann JF, Lee H, Li YJ, Tripathi SC, Yue C, et al. JAK/STAT3-regulated fatty acid β-oxidation is critical for breast cancer stem cell self-renewal and chemoresistance. Cell Metab. 2018;27:136–50.e5.
Sp N, Kang DY, Kim DH, Park JH, Lee HG, Kim HJ, et al. Nobiletin inhibits CD36-dependent tumor angiogenesis, migration, invasion, and sphere formation through the Cd36/Stat3/Nf-Κb signaling axis. Nutrients. 2018;10:772.
Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–5.
Acknowledgements
This study was supported by the Wenzhou Municipal Science and Technology Bureau (Y2020153), National Natural Science Foundation of China (81772628, 81703310, 82072685), Zhejiang-Germany Interdisciplinary Joint Laboratory of Hepatobiliary-Pancreatic Tumour and Bioengineering, and the Research Foundation of National Health Commission of China–Major Medical and Health Technology Project for Zhejiang Province (WKJ-ZJ-1706).
Author information
Authors and Affiliations
Contributions
GC, JLX and YW conceived and designed this research; TD, JGZ and YFT performed most of the experiments and summarized the results; ZYC, BJH and JCL performed some experiments and provided the data, BC, JYJ and BYG analysed the data; LMD, HTY, BFZ, TZ, ZHS, YFS and ZPY contributed reagents/materials/analysis tools; TD wrote the original manuscript, RZL and YPJ revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Study approval
All experiments involving animals were approved by the Animal Care and Use Committee of the First Affiliated Hospital of Wenzhou Medical University and were performed in accordance with the approved protocols. The HCC tumour samples, paraffin sections and blood samples used in the research were obtained from the First Affiliated Hospital of Wenzhou Medical University, and experiments involving human-derived samples were conducted with the approval of the ethics committee of the First Affiliated Hospital of Wenzhou Medical University. All patients provided written informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Deng, T., Zhao, J., Tong, Y. et al. Crosstalk between endothelial progenitor cells and HCC through periostin/CCL2/CD36 supports formation of the pro-metastatic microenvironment in HCC. Oncogene 43, 944–961 (2024). https://doi.org/10.1038/s41388-024-02960-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-024-02960-2