Abstract
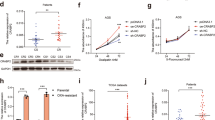
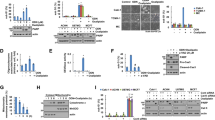
Continuous administration of oxaliplatin, the most widely used first-line chemotherapy drug for colorectal cancer (CRC), eventually leads to drug resistance. Increasing the sensitivity of CRC cells to oxaliplatin is a key strategy to overcome this issue. Impairment of mitochondrial function is a pivotal mechanism determining the sensitivity of CRC to oxaliplatin. We discovered an inverse correlation between Translocase of Outer Mitochondrial Membrane 20 (TOMM20) and oxaliplatin sensitivity as well as an inverse relationship between TOMM20 and HECT, UBA, and WWE domain containing E3 ligase 1 (HUWE1) expression in CRC. For the first time, we demonstrated that HUWE1 ubiquitinates TOMM20 directly and also regulates TOMM20 degradation via the PARKIN-mediated pathway. Furthermore, we showed that overexpression of HUWE1 in CRC cells has a negative effect on mitochondrial function, including the generation of ATP and maintenance of mitochondrial membrane potential, leading to increased production of ROS and apoptosis. This effect was amplified when cells were treated simultaneously with oxaliplatin. Our study conclusively shows that TOMM20 is a novel target of HUWE1. Our findings indicate that HUWE1 plays a critical role in regulating oxaliplatin sensitivity by degrading TOMM20 and inducing mitochondrial damage in CRC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All data supporting the findings of this study can be freely accessed by any researcher for non-commercial purposes upon reasonable request.
References
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48.
Ciombor KK, Wu C, Goldberg RM. Recent therapeutic advances in the treatment of colorectal cancer. Annu Rev Med. 2015;66:83–95.
Kuipers EJ, Grady WM, Lieberman D, Seufferlein T, Sung JJ, Boelens PG, et al. Colorectal cancer. Nat Rev Dis Primers. 2015;1:15065.
Marschner N, Arnold D, Engel E, Hutzschenreuter U, Rauh J, Freier W, et al. Oxaliplatin-based first-line chemotherapy is associated with improved overall survival compared to first-line treatment with irinotecan-based chemotherapy in patients with metastatic colorectal cancer - Results from a prospective cohort study. Clin Epidemiol. 2015;7:295–303.
Marin JJ, Sanchez de Medina F, Castaño B, Bujanda L, Romero MR, Martinez-Augustin O, et al. Chemoprevention, chemotherapy, and chemoresistance in colorectal cancer. Drug Metab Rev. 2012;44:148–72.
Martinez-Balibrea E, Martínez-Cardús A, Ginés A, Ruiz de Porras V, Moutinho C, Layos L, et al. Tumor-related molecular mechanisms of oxaliplatin resistance. Mol Cancer Ther. 2015;14:1767–76.
Chen Y, Deng G, Fu Y, Han Y, Guo C, Yin L, et al. FOXC2 Promotes oxaliplatin resistance by inducing epithelial-mesenchymal transition via MAPK/ERK signaling in colorectal cancer. Onco Targets Ther. 2020;13:1625–35.
Shen X, Zhang Y, Xu Z, Gao H, Feng W, Li W, et al. KLF5 inhibition overcomes oxaliplatin resistance in patient-derived colorectal cancer organoids by restoring apoptotic response. Cell Death Dis. 2022;13:303.
Yin Y, Li S, Liang X, Li K, Xie M, Hu B. Construction and validation of an oxaliplatin-resistant gene signature in colorectal cancer patients who underwent chemotherapy. Pharmaceuticals. 2022;15:1139.
Vyas S, Zaganjor E, Haigis MC. Mitochondria and cancer. Cell. 2016;166:555–66.
Gourdier I, Crabbe L, Andreau K, Pau B, Kroemer G. Oxaliplatin-induced mitochondrial apoptotic response of colon carcinoma cells does not require nuclear DNA. Oncogene. 2004;23:7449–57.
Leo M, Schmitt LI, Küsterarent P, Kutritz A, Rassaf T, Kleinschnitz C, et al. Platinum-based drugs cause mitochondrial dysfunction in cultured dorsal root ganglion neurons. Int J Mol Sci. 2020;21:8636.
Bahar E, Han SY, Kim JY, Yoon H. Chemotherapy resistance: role of mitochondrial and autophagic components. Cancers. 2022;14:1462.
Jin P, Jiang J, Zhou L, Huang Z, Nice EC, Huang C, et al. Mitochondrial adaptation in cancer drug resistance: prevalence, mechanisms, and management. J Hematol Oncol. 2022;15:97.
Yano M, Terada K, Mori M. Mitochondrial import receptors Tom20 and Tom22 have chaperone-like activity. J Biol Chem. 2004;279:10808–13.
Park SH, Lee AR, Choi K, Joung S, Yoon JB, Kim S. TOMM20 as a potential therapeutic target of colorectal cancer. BMB Rep. 2019;52:712–7.
Franco-Iborra S, Cuadros T, Parent A, Romero-Gimenez J, Vila M, Perier C. Defective mitochondrial protein import contributes to complex I-induced mitochondrial dysfunction and neurodegeneration in Parkinson’s disease. Cell Death Dis. 2018;9:1122.
Roche ME, Lin Z, Whitaker-Menezes D, Zhan T, Szuhai K, Bovee JVMG, et al. Translocase of the outer mitochondrial membrane complex subunit 20 (TOMM20) facilitates cancer aggressiveness and therapeutic resistance in chondrosarcoma. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165962.
Gong X, Du D, Deng Y, Zhou Y, Sun L, Yuan S. The structure and regulation of the E3 ubiquitin ligase HUWE1 and its biological functions in cancer. Invest New Drugs. 2020;38:515–24.
Kao SH, Wu HT, Wu KJ. Ubiquitination by HUWE1 in tumorigenesis and beyond. J Biomed Sci. 2018;25:67.
Zhong Q, Gao W, Du F, Wang X. Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell. 2005;121:1085–95.
Wang X, Lu G, Li L, Yi J, Yan K, Wang Y, et al. HUWE1 interacts with BRCA1 and promotes its degradation in the ubiquitin-proteasome pathway. Biochem Biophys Res Commun. 2014;444:549–54.
Yang D, Cheng D, Tu Q, Yang H, Sun B, Yan L, et al. HUWE1 controls the development of non-small cell lung cancer through down-regulation of p53. Theranostics. 2018;8:3517–29.
Leboucher GP, Tsai YC, Yang M, Shaw KC, Zhou M, Veenstra TD, et al. Stress-induced phosphorylation and proteasomal degradation of mitofusin 2 facilitates mitochondrial fragmentation and apoptosis. Mol Cell. 2012;47:547–57.
Di Rita A, Peschiaroli A, D Acunzo P, Strobbe D, Hu Z, et al. HUWE1 E3 ligase promotes PINK1/PARKIN-independent mitophagy by regulating AMBRA1 activation via IKKα. Nat Commun. 2018;9:3755.
Yoshii SR, Kishi C, Ishihara N, Mizushima N. Parkin mediates proteasome-dependent protein degradation and rupture of the outer mitochondrial membrane. J Biol Chem. 2011;286:19630–40.
Hollville E, Carroll RG, Cullen SP, Martin SJ. Bcl-2 family proteins participate in mitochondrial quality control by regulating Parkin/PINK1-dependent mitophagy. Mol Cell. 2014;55:451–66.
Chen B, Das NK, Talukder I, Singhal R, Castillo C, Andren A, et al. PTEN-induced kinase PINK1 supports colorectal cancer growth by regulating the labile iron pool. J Biol Chem. 2023;299:104691.
Ma Z, Liu Z, Li X, Zhang H, Han D, Xiong W, et al. Metformin collaborates with PINK1/Mfn2 overexpression to prevent cardiac injury by improving mitochondrial function. Biology. 2023;12:582.
Hall JR, Kow E, Nevis KR, Lu CK, Luce KS, Zhong Q, et al. Cdc6 stability is regulated by the Huwe1 ubiquitin ligase after DNA damage. Mol Biol Cell. 2007;18:3340–50.
Ma W, Zhao P, Zang L, Zhang K, Liao H, Hu Z. Tumour suppressive function of HUWE1 in thyroid cancer. J Biosci. 2016;41:395–405.
Wenmaekers S, Viergever BJ, Kumar G, Kranenburg O, Black PC, Daugaard M, et al. A potential role for HUWE1 in modulating cisplatin sensitivity. Cells. 2021;10:1262.
Cassidy KB, Bang S, Kurokawa M, Gerber SA. Direct regulation of Chk1 protein stability by E3 ubiquitin ligase HUWE1. FEBS J. 2020;287:1985–99.
Jeong S, Kim BG, Kim DY, Kim BR, Kim JL, Park SH, et al. Cannabidiol overcomes oxaliplatin resistance by enhancing NOS3- and SOD2-induced autophagy in human colorectal cancer cells. Cancers. 2019;11:781.
Sun W, Ge Y, Cui J, Yu Y, Liu B. Scutellarin resensitizes oxaliplatin-resistant colorectal cancer cells to oxaliplatin treatment through inhibition of PKM2. Mol Ther Oncolytics. 2021;21:87–97.
Yan C, Li TS. Dual role of mitophagy in cancer drug resistance. Anticancer Res. 2018;38:617–21.
Guan Y, Wang Y, Li B, Shen K, Li Q, Ni Y, et al. Mitophagy in carcinogenesis, drug resistance and anticancer therapeutics. Cancer Cell Int. 2021;21:350.
Yan C, Luo L, Guo CY, Goto S, Urata Y, Shao JH, et al. Doxorubicin-induced mitophagy contributes to drug resistance in cancer stem cells from HCT8 human colorectal cancer cells. Cancer Lett. 2017;388:34–42.
Okon IS, Zou MH. Mitochondrial ROS and cancer drug resistance: implications for therapy. Pharmacol Res. 2015;100:170–4.
Chan NC, Salazar AM, Pham AH, Sweredoski MJ, Kolawa NJ, Graham RL, et al. Broad activation of the ubiquitin-proteasome system by Parkin is critical for mitophagy. Hum Mol Genet. 2011;20:1726–37.
Boyle KA, Van Wickle J, Hill RB, Marchese A, Kalyanaraman B, Dwinell MB. Mitochondria-targeted drugs stimulate mitophagy and abrogate colon cancer cell proliferation. J Biol Chem. 2018;293:14891–904.
Wang MM, Xu FJ, Su Y, Geng Y, Qian XT, Xue XL, et al. A new strategy to fight metallodrug resistance: mitochondria-relevant treatment through mitophagy to inhibit metabolic adaptations of cancer cells. Angew Chem Int Ed Engl. 2022;61:e202203843.
Myant KB, Cammareri P, Hodder MC, Wills J, Von Kriegsheim A, Győrffy B, et al. HUWE1 is a critical colonic tumor suppressor gene that prevents MYC signaling, DNA damage accumulation, and tumor initiation. EMBO Mol Med. 2017;9:181–97.
Fiorillo M, Ózsvári B, Sotgia F, Lisanti MP. High ATP production fuels cancer drug resistance and metastasis: implications for mitochondrial ATP depletion therapy. Front Oncol. 2021;11:740720.
Liu S, Cong Y, Wang D, Sun Y, Deng L, Liu Y, et al. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Reports. 2013;2:78–91.
Recasens A, Munoz L. Targeting cancer cell dormancy. Trends Pharmacol Sci. 2019;40:128–41.
Xiong Y, Kotake Y. No exit strategy? No problem: APC inhibits beta-catenin inside the nucleus. Genes Dev. 2006;20:637–42.
Leppert M, Burt R, Hughes JP, Samowitz W, Nakamura Y, Woodward S, et al. Genetic analysis of an inherited predisposition to colon cancer in a family with a variable number of adenomatous polyps. N Engl J Med. 1990;322:904–8.
Segditsas S, Tomlinson I. Colorectal cancer and genetic alterations in the Wnt pathway. Oncogene. 2006;25:7531–7.
Schatoff EM, Leach BI, Dow LE. Wnt signaling and colorectal cancer. Curr Colorectal Cancer Rep. 2017;13:101–10.
de Sousa E Melo F, Vermeulen L. Wnt signaling in cancer stem cell biology. Cancers. 2016;8:60.
Tanaka H, Kawaguchi M, Shoda S, Miyoshi T, Iwasaki R, Hyodo F, et al. Nuclear accumulation of β-Catenin in cancer stem cell radioresistance and stemness in human colon cancer. Anticancer Res. 2019;39:6575–83.
Dominguez-Brauer C, Khatun R, Elia AJ, Thu KL, Ramachandran P, Baniasadi SP, et al. E3 ubiquitin ligase Mule targets β-catenin under conditions of hyperactive Wnt signaling. Proc Natl Acad Sci USA. 2017;114:E1148–E1157.
Rodríguez-Fanjul V, Guerrero-López R, Fernández-Varas B, Perona R, Sastre-Perona A, Sastre L. Comparison of colorectal cancer stem cells and oxaliplatin-resistant cells unveils functional similarities. Cells. 2022;11:511.
Boye C, Arpag S, Francis M, DeClemente S, West A, Heller R, et al. Reduction of plasmid vector backbone length enhances reporter gene expression. Bioelectrochemistry. 2022;144:107981.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government. (MSIT) (2022R1F1A1066987, RS-2023-00273665).
Author information
Authors and Affiliations
Contributions
CL and SKY conceived the study and designed the experiment. CL and SHP performed the experiment and analyzed the data. CL and SKY interpreted and discussed the data. CL wrote the manuscript. SKY revised the manuscript and supervised the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, C., Park, SH. & Yoon, S.K. The E3 ligase HUWE1 increases the sensitivity of CRC to oxaliplatin through TOMM20 degradation. Oncogene 43, 636–649 (2024). https://doi.org/10.1038/s41388-023-02928-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-023-02928-8